Nuclear atrophy of retinal ganglion cells precedes the bax-dependent stage of apoptosis
- PMID: 23422829
- PMCID: PMC3626519
- DOI: 10.1167/iovs.11-9310
Nuclear atrophy of retinal ganglion cells precedes the bax-dependent stage of apoptosis
Abstract
Purpose: Retinal ganglion cells atrophy during the execution of the intrinsic apoptotic program. This process, which has been termed the apoptotic volume decrease (AVD) in other cell types, has not been well-characterized in ganglion cells.
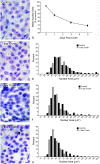
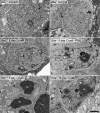
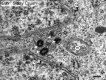
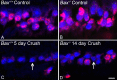
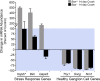
Methods: Acute optic nerve crush was used to examine neuronal atrophy in the ganglion cell layer in wild-type and Bax-deficient mice. Nuclear size was measured from retinal wholemounts. Heterochromatin formation was assessed using transmission electron microscopy, whereas histone H4 acetylation was monitored using immunofluoresence. Ganglion cell and retinal transcript abundance was measured using quantitative PCR.
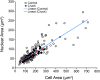
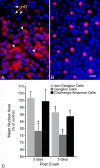
Results: Nuclear and soma sizes linearly correlated in both control and damaged retinas. Cells in wild-type mice exhibited nuclear atrophy within 1 day after optic nerve damage. Three days after crush, nuclear atrophy was restricted to ganglion cells identified by retrograde labeling, while amacrine cells also exhibited some atrophy by 5 days. Similar kinetics of nuclear atrophy were observed in cells deficient for the essential proapoptotic gene Bax. Bax-deficient cells also exhibited other nuclear changes common in wild-type cells, including the deacetylation of histones, formation of heterochromatin, and the silencing of ganglion cell-specific gene expression.
Conclusions: Retinal ganglion cell somas and nuclei undergo the AVD in response to optic nerve damage. Atrophy is rapid and precedes the Bax-dependent committed step of the intrinsic apoptotic pathway.
Conflict of interest statement
Disclosure:
Figures

References
-
- Nickells RW. Retinal ganglion cell death in glaucoma: the how, the why, and the maybe. J Glaucoma. 1996; 5: 345–356 - PubMed
-
- Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007; 42: 278–287 - PubMed
-
- Morrison JC, Cepurna WO, Jia L, Aubert J, Johnson EC. Mechanism of focal optic nerve injury from elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2002; 43: U813
-
- Morrison JC, Johnson EC, Cepurna WA, Jia L. Understanding mechanisms of pressure-induced optic nerve damage. Prog Retin Eye Res. 2005; 24: 217–240 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

