Randomized study combining interferon and glatiramer acetate in multiple sclerosis
- PMID: 23424159
- PMCID: PMC3631288
- DOI: 10.1002/ana.23863
Randomized study combining interferon and glatiramer acetate in multiple sclerosis
Abstract
Objective: A double-blind, randomized, controlled study was undertaken to determine whether combined use of interferon β-1a (IFN) 30 μg intramuscularly weekly and glatiramer acetate (GA) 20 mg daily is more efficacious than either agent alone in relapsing-remitting multiple sclerosis.
Methods: A total of 1,008 participants were randomized and followed until the last participant enrolled completed 3 years. The primary endpoint was reduction in annualized relapse rate utilizing a strict definition of relapse. Secondary outcomes included time to confirmed disability, Multiple Sclerosis Functional Composite (MSFC) score, and magnetic resonance imaging (MRI) metrics.
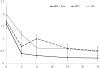
Results: Combination IFN+GA was not superior to the better of the single agents (GA) in risk of relapse. Both the combination therapy and GA were significantly better than IFN in reducing the risk of relapse. The combination was not better than either agent alone in lessening confirmed Expanded Disability Status Scale progression or change in MSFC over 36 months. The combination was superior to either agent alone in reducing new lesion activity and accumulation of total lesion volumes. In a post hoc analysis, combination therapy resulted in a higher proportion of participants attaining disease activity-free status (DAFS) compared to either single arm, driven by the MRI results.
Interpretation: Combining the 2 most commonly prescribed therapies for multiple sclerosis did not produce a significant clinical benefit over 3 years. An effect was seen on some MRI metrics. In a test of comparative efficacy, GA was superior to IFN in reducing the risk of exacerbation. The extension phase for CombiRx will address whether the observed differences in MRI and DAFS findings predict later clinical differences.
Trial registration: ClinicalTrials.gov NCT00211887.
Copyright © 2013 American Neurological Association.
Figures










Comment in
-
Multiple sclerosis: monotherapy rules.Ann Neurol. 2013 Mar;73(3):A5-6. doi: 10.1002/ana.23886. Ann Neurol. 2013. PMID: 23596013 No abstract available.
References
-
- Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol. 2009;65:239–248. - PubMed
-
- The IFNB MS study group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:655–661. - PubMed
-
- Conway D, Cohen JA. Combination therapy in multiple sclerosis. Lancet Neurol. 2010;9:299–308. - PubMed
-
- Graber JJ, McGraw CA, Kimbrough D, et al. Overlapping and distinct mechanisms of action of multiple sclerosis therapies. Clin Neurol Neurosurg. 2010;112:583–591. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

