A dual strategy to cope with high light in Chlamydomonas reinhardtii
- PMID: 23424243
- PMCID: PMC3608777
- DOI: 10.1105/tpc.112.108274
A dual strategy to cope with high light in Chlamydomonas reinhardtii
Abstract
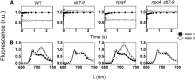
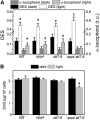
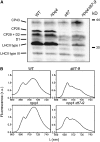
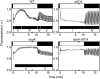
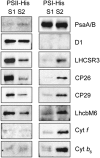
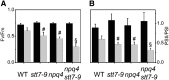
Absorption of light in excess of the capacity for photosynthetic electron transport is damaging to photosynthetic organisms. Several mechanisms exist to avoid photodamage, which are collectively referred to as nonphotochemical quenching. This term comprises at least two major processes. State transitions (qT) represent changes in the relative antenna sizes of photosystems II and I. High energy quenching (qE) is the increased thermal dissipation of light energy triggered by lumen acidification. To investigate the respective roles of qE and qT in photoprotection, a mutant (npq4 stt7-9) was generated in Chlamydomonas reinhardtii by crossing the state transition-deficient mutant (stt7-9) with a strain having a largely reduced qE capacity (npq4). The comparative phenotypic analysis of the wild type, single mutants, and double mutants reveals that both state transitions and qE are induced by high light. Moreover, the double mutant exhibits an increased photosensitivity with respect to the single mutants and the wild type. Therefore, we suggest that besides qE, state transitions also play a photoprotective role during high light acclimation of the cells, most likely by decreasing hydrogen peroxide production. These results are discussed in terms of the relative photoprotective benefit related to thermal dissipation of excess light and/or to the physical displacement of antennas from photosystem II.
Figures


References
-
- Allen J.F. (1992). Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098: 275–335 - PubMed
-
- Aro E.M., Ohad I. (2003). Redox regulation of thylakoid protein phosphorylation. Antioxid. Redox Signal. 5: 55–67 - PubMed
-
- Aro E.M., Virgin I., Andersson B. (1993). Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143: 113–134 - PubMed
-
- Bailleul B., Cardol P., Breyton C., Finazzi G. (2010a). Electrochromism: A useful probe to study algal photosynthesis. Photosynth. Res. 106: 179–189 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

