Benzyl N-[(Z)-(1-methyl-2-sulfanyl-propyl-idene)amino]-carbamodithio-ate
- PMID: 23424463
- PMCID: PMC3569240
- DOI: 10.1107/S1600536812051008
Benzyl N-[(Z)-(1-methyl-2-sulfanyl-propyl-idene)amino]-carbamodithio-ate
Abstract
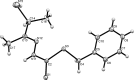
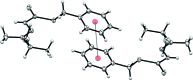
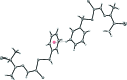
The title compound, C(12)H(16)N(2)S(3), was obtained by the condensation reaction of S-benzyl dithio-carbazate and 3-mercaptobutan-2-one. The phenyl ring and thiol (SH) group are approximately perpendicular [S-C-C-C and N-C-C-S torsion angles = 67.8 (3) and 116.9 (2)°, respectively] to the rest of the mol-ecule. In the crystal, mol-ecules are linked by weak S-H⋯S and N-H⋯S hydrogen bonds, π-π inter-actions between the benzene rings [centroid-centroid distance = 3.823 (2) Å] and C-H⋯π inter-actions.
Figures

References
-
- Agilent (2011). CrysAlis PRO Agilent Technologies UK Ltd, Yarnton, England.
-
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
-
- Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K. & Watkin, D. J. (2003). J. Appl. Cryst. 36, 1487.
-
- Hossain, E., Alam, M., Ali, M., Nazimuddin, M., Smith, E. & Hynes, C. (1996). Polyhedron, 15, 973–980.
-
- How, F. N.-F., Watkin, D. J., Crouse, K. A. & Tahir, M. I. M. (2007). Acta Cryst. E63, o3023–o3024.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
