N-(5-Amino-1H-1,2,4-triazol-3-yl)pyridine-2-carboxamide
- PMID: 23424508
- PMCID: PMC3569762
- DOI: 10.1107/S1600536813000123
N-(5-Amino-1H-1,2,4-triazol-3-yl)pyridine-2-carboxamide
Abstract
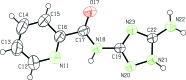
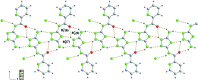
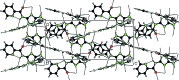
The title compound, C(8)H(8)N(6)O, was obtained by the reaction of 3,5-diamino-1,2,4-triazole with ethyl 2-picolinate in a glass oven. The dihedral angles formed between the plane of the amide group and the pyridine and triazole rings are 11.8 (3) and 5.8 (3)°, respectively. In the crystal, an extensive system of classical N-H⋯N and N-H⋯O hydrogen bonds generate an infinite three-dimensional network.
Figures

References
-
- Aromí, G., Barrios, L., Roubeuau, O. & Gámez, P. (2011). Coord. Chem. Rev. 255, 485–546.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
-
- Brandenburg, K. & Putz, H. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
-
- Chernyshev, V. M., Rakitov, V. A., Taranushich, V. A. & Blinov, V. V. (2005). Chem. Heterocycl. Compd, 41, 1139–1146.
LinkOut - more resources
Full Text Sources
Other Literature Sources
