(6bS*,14R*,14aR*)-Methyl 14-(4-methyl-phen-yl)-7-oxo-6b,6c,7,12b,14,14a-hexa-hydro-1H-pyrano[3,2-c:5,4-c']dichromene-14a-carboxyl-ate
- PMID: 23424543
- PMCID: PMC3569797
- DOI: 10.1107/S1600536813001244
(6bS*,14R*,14aR*)-Methyl 14-(4-methyl-phen-yl)-7-oxo-6b,6c,7,12b,14,14a-hexa-hydro-1H-pyrano[3,2-c:5,4-c']dichromene-14a-carboxyl-ate
Abstract
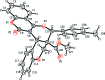
In the title compound, C(28)H(22)O(6), the chromeno ring system is almost planar, with a dihedral angle between the mean planes of the pyran and benzene rings of 1.87 (8)°. The pyran ring bearing the methyl-phenyl substituent has a half-chair conformation while the other pyran ring has an envelope conformation with the tetra-substituted C atom as the flap. The benzene ring of the chromeno ring system is inclined to the benzene ring fused to the latter pyran ring by 74.66 (9)°. These aromatic rings are inclined to the 4-methyl-phenyl ring by 52.67 (9) and 66.63 (10)°, respectively. In the crystal, mol-ecules are linked via C-H⋯O hydrogen bonds, forming a two-dimensional network parallel to the bc plane.
Figures

References
-
- Brooks, G. T. (1998). Pestic. Sci. 22, 41–50.
-
- Bruker (2008). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cai, S. X. (2007). Recent Patents Anticancer Drug Discov. 2, 79–101. - PubMed
-
- Cai, S. X. (2008). Bioorg. Med. Chem. Lett. 18, 603–607. - PubMed
-
- Cai, S. X., Drewe, J. & Kasibhatla, S. (2006). Curr. Med. Chem. 13, 2627–2644. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
