Binding of DC-SIGN to the hemagglutinin of influenza A viruses supports virus replication in DC-SIGN expressing cells
- PMID: 23424649
- PMCID: PMC3570528
- DOI: 10.1371/journal.pone.0056164
Binding of DC-SIGN to the hemagglutinin of influenza A viruses supports virus replication in DC-SIGN expressing cells
Abstract
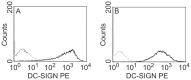
Dendritic cells express lectins receptors, like DC-SIGN, which allow these cells to sense glycans that are present on various bacterial and viral pathogens. Interaction of DC-SIGN with carbohydrate moieties induces maturation of dendritic cells and promotes endocytosis of pathogens which is an important property of these professional antigen presenting cells. Uptake of pathogens by dendritic cells may lead to cross-presentation of antigens or infection of these cells, which ultimately results in activation of virus-specific T cells in draining lymph nodes. Little is known about the interaction of DC-SIGN with influenza A viruses. Here we show that a virus with a non-functional receptor binding site in its hemagglutinin, can replicate in cells expressing DC-SIGN. Also in the absence of sialic acids, which is the receptor for influenza A viruses, these viruses replicate in DC-SIGN expressing cells including human dendritic cells. Furthermore, the efficiency of DC-SIGN mediated infection is dependent on the extent of glycosylation of the viral hemagglutinin.
Conflict of interest statement
Figures



References
-
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, et al. (2000) DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100: 587–597. - PubMed
-
- Feinberg H, Mitchell DA, Drickamer K, Weis WI (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294: 2163–2166. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

