8-BuS-ATP derivatives as specific NTPDase1 inhibitors
- PMID: 23425137
- PMCID: PMC3632248
- DOI: 10.1111/bph.12135
8-BuS-ATP derivatives as specific NTPDase1 inhibitors
Abstract
Background and purpose: Ectonucleotidases control extracellular nucleotide levels and consequently, their (patho)physiological responses. Among these enzymes, nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), -2, -3 and -8 are the major ectonucleotidases responsible for nucleotide hydrolysis at the cell surface under physiological conditions, and NTPDase1 is predominantly located at the surface of vascular endothelial cells and leukocytes. Efficacious inhibitors of NTPDase1 are required to modulate responses induced by nucleotides in a number of pathological situations such as thrombosis, inflammation and cancer.
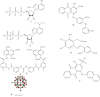
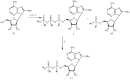
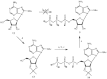
Experimental approach: Here, we present the synthesis and enzymatic characterization of five 8-BuS-adenine nucleotide derivatives as potent and selective inhibitors of NTPDase1.
Key results: The compounds 8-BuS-AMP, 8-BuS-ADP and 8-BuS-ATP inhibit recombinant human and mouse NTPDase1 by mixed type inhibition, predominantly competitive with Ki values <1 μM. In contrast to 8-BuS-ATP which could be hydrolyzed by other NTPDases, the other BuS derivatives were resistant to hydrolysis by either NTPDase1, -2, -3 or -8. 8-BuS-AMP and 8-BuS-ADP were the most potent and selective inhibitors of NTPDase1 expressed in human umbilical vein endothelial cells as well as in situ in human and mouse tissues. As expected, as a result of their inhibition of recombinant human NTPDase1, 8-BuS-AMP and 8-BuS-ADP impaired the ability of this enzyme to block platelet aggregation. Importantly, neither of these two inhibitors triggered platelet aggregation nor prevented ADP-induced platelet aggregation, in support of their inactivity towards P2Y1 and P2Y12 receptors.
Conclusions and implications: The 8-BuS-AMP and 8-BuS-ADP have therefore potential to serve as drugs for the treatment of pathologies regulated by NTPDase1.
© 2013 The Authors. British Journal of Pharmacology © 2013 The British Pharmacological Society.
Figures
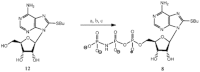




References
-
- Atkinson B, Dwyer K, Enjyoji K, Robson SC. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential as therapeutic targets. Blood Cells Mol Dis. 2006;36:217–222. - PubMed
-
- Baqi Y, Atzler K, Kose M, Glanzel M, Muller CE. High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J Med Chem. 2009;52:3784–3793. - PubMed
-
- Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171:266–270. - PubMed
-
- Belli SI, Goding JW. Biochemical characterization of human PC-1, an enzyme possessing alkaline phosphodiesterase I and nucleotide pyrophosphatase activities. Eur J Biochem. 1994;226:433–443. - PubMed
-
- Bigonnesse F, Lévesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJG, et al. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

