Effect of store and forward teledermatology on quality of life: a randomized controlled trial
- PMID: 23426111
- PMCID: PMC3726199
- DOI: 10.1001/2013.jamadermatol.380
Effect of store and forward teledermatology on quality of life: a randomized controlled trial
Abstract
Importance: Although research on quality of life and dermatologic conditions is well represented in the literature, information on teledermatology's effect on quality of life is virtually absent.
Objective: To determine the effect of store and forward teledermatology on quality of life.
Design: Two-site, parallel-group, superiority randomized controlled trial.
Setting: Dermatology clinics and affiliated sites of primary care at 2 US Department of Veterans Affairs medical facilities.
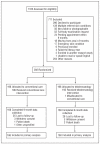
Participants: Patients being referred to a dermatology clinic were randomly assigned, stratified by site, to teledermatology or the conventional consultation process. Among the 392 patients who met the inclusion criteria and were randomized, 326 completed the allocated intervention and were included in the analysis.
Interventions: Store and forward teledermatology (digital images and a standardized history) or conventional text-based consultation processes were used to manage the dermatology consultations. Patients were followed up for 9 months.
Main outcome measures: The primary end point was change in Skindex-16 scores, a skin-specific quality-of-life instrument, between baseline and 9 months. A secondary end point was change in Skindex-16 scores between baseline and 3 months.
Results: Patients in both randomization groups demonstrated a clinically significant improvement in Skindex-16 scores between baseline and 9 months with no significant difference by randomization group (P = .66, composite score). No significant difference in Skindex-16 scores by randomization group between baseline and 3 months was found (P = .39, composite score).
Conclusions: Compared with the conventional consultation process, store and forward teledermatology did not result in a statistically significant difference in skin-related quality of life at 3 or 9 months after referral.
Trial registration: clinicaltrials.gov Identifier: NCT00488293.
Figures
References
-
- Chren MM. Giving “scale” new meaning in dermatology: measurement matters. Arch Dermatol. 2000;136(6):788–790. - PubMed
-
- Chren MM. Understanding research about quality of life and other health outcomes. J Cutan Med Surg. 1999;3(6):312–316. - PubMed
-
- Finlay AY, Khan GK, Luscombe DK, Salek MS. Validation of Sickness Impact Profile and Psoriasis Disability Index in psoriasis. Br J Dermatol. 1990;123(6):751–756. - PubMed
-
- Lundberg L, Johannesson M, Silverdahl M, Hermansson C, Lindberg M. Quality of life, health-state utilities and willingness to pay in patients with psoriasis and atopic eczema. Br J Dermatol. 1999;141(6):1067–1075. - PubMed
-
- Mallon E, Newton JN, Klassen A, Stewart-Brown SL, Ryan TJ, Finlay AY. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140(4):672–676. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

