IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality
- PMID: 23426178
- PMCID: PMC3582130
- DOI: 10.1172/JCI64931
IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality
Abstract
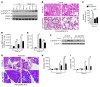
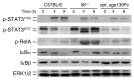
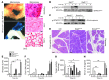
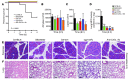
Acute lung injury (ALI) is an inflammatory disease with a high mortality rate. Although typically seen in individuals with sepsis, ALI is also a major complication in severe acute pancreatitis (SAP). The pathophysiology of SAP-associated ALI is poorly understood, but elevated serum levels of IL-6 is a reliable marker for disease severity. Here, we used a mouse model of acute pancreatitis-associated (AP-associated) ALI to determine the role of IL-6 in ALI lethality. Il6-deficient mice had a lower death rate compared with wild-type mice with AP, while mice injected with IL-6 were more likely to develop lethal ALI. We found that inflammation-associated NF-κB induced myeloid cell secretion of IL-6, and the effects of secreted IL-6 were mediated by complexation with soluble IL-6 receptor, a process known as trans-signaling. IL-6 trans-signaling stimulated phosphorylation of STAT3 and production of the neutrophil attractant CXCL1 in pancreatic acinar cells. Examination of human samples revealed expression of IL-6 in combination with soluble IL-6 receptor was a reliable predictor of ALI in SAP. These results demonstrate that IL-6 trans-signaling is an essential mediator of ALI in SAP across species and suggest that therapeutic inhibition of IL-6 may prevent SAP-associated ALI.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

