Expanding proteostasis by membrane trafficking networks
- PMID: 23426524
- PMCID: PMC3685892
- DOI: 10.1101/cshperspect.a013383
Expanding proteostasis by membrane trafficking networks
Abstract
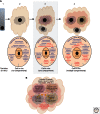
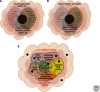
The folding biology common to all three kingdoms of life (Archaea, Bacteria, and Eukarya) is proteostasis. The proteostasis network (PN) functions as a "cloud" to generate, protect, and degrade the proteome. Whereas microbes (Bacteria, Archaea) have a single compartment, Eukarya have numerous subcellular compartments. We examine evidence that Eukarya compartments use coat, tether, and fusion (CTF) membrane trafficking components to form an evolutionarily advanced arm of the PN that we refer to as the "trafficking PN" (TPN). We suggest that the TPN builds compartments by generating a mosaic of integrated cargo-specific trafficking signatures (TRaCKS). TRaCKS control the temporal and spatial features of protein-folding biology based on the Anfinsen principle that the local environment plays a critical role in managing protein structure. TPN-generated endomembrane compartments apply a "quinary" level of structural control to modify the secondary, tertiary, and quaternary structures defined by the primary polypeptide-chain sequence. The development of Anfinsen compartments provides a unifying foundation for understanding the purpose of endomembrane biology and its capacity to drive extant Eukarya function and diversity.
Figures


References
-
- Allan BB, Balch WE 1999. Protein sorting by directed maturation of Golgi compartments. Science 285: 63–66 - PubMed
-
- Allan BB, Moyer BD, Balch WE 2000. Rab1 recruitment of p115 into a cis-SNARE complex: Programming budding COPII vesicles for fusion. Science 289: 444–448 - PubMed
-
- Anfinsen CB 1973. Principles that govern the folding of protein chains. Science 181: 223–230 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
