Midbrain-derived neurotrophins support survival of immature striatal projection neurons
- PMID: 23426664
- PMCID: PMC3711532
- DOI: 10.1523/JNEUROSCI.3687-12.2013
Midbrain-derived neurotrophins support survival of immature striatal projection neurons
Abstract
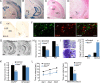
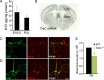
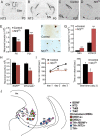
Neuronal death occurs at several stages during embryogenesis and early postnatal development; however, it is unknown how the survival of immature neurons at their origin is regulated before these cells migrate to their final destination. Striatal projection neurons, known as medium-sized spiny neurons (MSNs), in both the direct and indirect pathways are generated in the lateral ganglionic eminence (LGE). Here we report that brain-derived neurotrophic factor and neurotrophin-3 are anterogradely transported from midbrain dopaminergic neurons and support the survival of immature MSNs of the indirect and direct pathways, respectively, in the developing mouse striatum and LGE. These results reveal a novel mode of neurotrophic action in the nervous system by linking neurotrophins to the survival of immature neurons at their origin, while also suggesting that innervating neurons may control the size of their targeting neuronal population in the brain.
Figures

References
-
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. - PubMed
-
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
