Spontaneous optic nerve compression in the osteopetrotic (op/op) mouse: a novel model of myelination failure
- PMID: 23426679
- PMCID: PMC3677540
- DOI: 10.1523/JNEUROSCI.4849-12.2013
Spontaneous optic nerve compression in the osteopetrotic (op/op) mouse: a novel model of myelination failure
Abstract
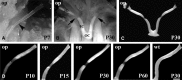
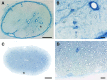
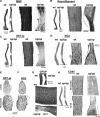
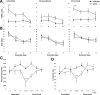
We report a focal disturbance in myelination of the optic nerve in the osteopetrotic (op/op) mouse, which results from a spontaneous compression of the nerve resulting from stenosis of the optic canal. The growth of the op/op optic nerve was significantly affected, being maximally suppressed at postnatal day 30 (P30; 33% of age matched control). Myelination of the nerve in the optic canal was significantly delayed at P15, and myelin was almost completely absent at P30. The size of nerves and myelination were conserved both in the intracranial and intraorbital segments at P30, suggesting that the axons in the compressed site are spared in all animals at P30. Interestingly, we observed recovery both in the nerve size and the density of myelinated axons at 7 months in almost half of the optic nerves examined, although some nerves lost axons and became atrophic. In vivo and ex vivo electrophysiological examinations of P30 op/op mice showed that nerve conduction was significantly delayed but not blocked with partial recovery in some mice by 7 months. Transcardial perfusion of FITC-labeled albumin suggested that local ischemia was at least in part the cause of this myelination failure. These results suggest that the primary abnormality is dysmyelination of the optic nerve in early development. This noninvasive model system will be a valuable tool to study the effects of nerve compression on the function and survival of oligodendrocyte progenitor cells/oligodendrocytes and axons and to explore the mechanism of redistribution of oligodendrocyte progenitor cells with compensatory myelination.
Figures






References
-
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. - PubMed
-
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992a;70:31–46. - PubMed
-
- Barres BA, Hart IK, Coles HC, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death in oligodendrocyte lineage. J Neurobiol. 1992b;10:42:49:1–10. - PubMed
-
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
