CCAAT enhancer binding protein δ plays an essential role in memory consolidation and reconsolidation
- PMID: 23426691
- PMCID: PMC3711718
- DOI: 10.1523/JNEUROSCI.1635-12.2013
CCAAT enhancer binding protein δ plays an essential role in memory consolidation and reconsolidation
Abstract
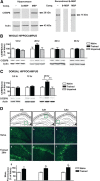
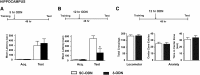
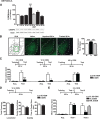
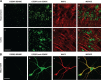
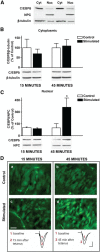
A newly formed memory is temporarily fragile and becomes stable through a process known as consolidation. Stable memories may again become fragile if retrieved or reactivated, and undergo a process of reconsolidation to persist and strengthen. Both consolidation and reconsolidation require an initial phase of transcription and translation that lasts for several hours. The identification of the critical players of this gene expression is key for understanding long-term memory formation and persistence. In rats, the consolidation of inhibitory avoidance (IA) memory requires gene expression in both the hippocampus and amygdala, two brain regions that process contextual/spatial and emotional information, respectively; IA reconsolidation requires de novo gene expression in the amygdala. Here we report that, after IA learning, the levels of the transcription factor CCAAT enhancer binding protein δ (C/EBPδ) are significantly increased in both the hippocampus and amygdala. These increases are essential for long-term memory consolidation, as their blockade via antisense oligodeoxynucleotide-mediated knockdown leads to memory impairment. Furthermore, C/EBPδ is upregulated and required in the amygdala for IA memory reconsolidation. C/EBPδ is found in nuclear, somatic, and dendritic compartments, and a dendritic localization of C/EBPδ mRNA in hippocampal neuronal cultures suggests that this transcription factor may be translated at synapses. Finally, the induction of long-term potentiation at CA3-CA1 synapses by tetanic stimuli in acute slices, a cellular model of long-term memory, leads to an accumulation of C/EBPδ in the nucleus. We conclude that the transcription factor C/EBPδ plays a critical role in memory consolidation and reconsolidation.
Figures

References
-
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. - PubMed
-
- Alberini CM, Ghirardi M, Huang YY, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann N Y Acad Sci. 1995;758:261–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
