Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice
- PMID: 23426919
- PMCID: PMC3688704
- DOI: 10.1002/jbmr.1894
Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice
Abstract
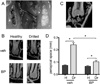
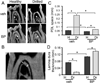
Osteonecrosis of the jaw (ONJ) is a well-recognized complication of antiresorptive medications, such as bisphosphonates (BPs). Although ONJ is most common after tooth extractions in patients receiving high-dose BPs, many patients do not experience oral trauma. Animal models using tooth extractions and high BP doses recapitulate several clinical, radiographic, and histologic findings of ONJ. We and others have reported on rat models of ONJ using experimental dental disease in the absence of tooth extraction. These models emphasize the importance of dental infection/inflammation for ONJ development. Here, we extend our original report in the rat, and present a mouse model of ONJ in the presence of dental disease. Mice were injected with high dose zoledronic acid and pulpal exposure of mandibular molars was performed to induce periapical disease. After 8 weeks, quantitative and qualitative radiographic and histologic analyses of mouse mandibles were done. Periapical lesions were larger in vehicle-treated versus BP-treated mice. Importantly, radiographic features resembling clinical ONJ, including thickening of the lamina dura, periosteal bone deposition, and increased trabecular density, were seen in the drilled site of BP-treated animals. Histologically, osteonecrosis, periosteal thickening, periosteal bone apposition, epithelial migration, and bone exposure were present in the BP-treated animals in the presence of periapical disease. No difference in tartrate-resistant acid phosphatase (TRAP)+ cell numbers was observed, but round, detached, and removed from the bone surface cells were present in BP-treated animals. Although 88% of the BP-treated animals showed areas of osteonecrosis in the dental disease site, only 33% developed bone exposure, suggesting that osteonecrosis precedes bone exposure. Our data further emphasize the importance of dental disease in ONJ development, provide qualitative and quantitative measures of ONJ, and present a novel mouse ONJ model in the absence of tooth extraction that should be useful in further exploring ONJ pathophysiological mechanisms.
Copyright © 2013 American Society for Bone and Mineral Research.
Conflict of interest statement
Dr. Tetradis has served as a paid consultant for and has received grant support from Amgen Inc. All other authors state that they do not have any conflicts of interest.
Figures






References
-
- Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. - PubMed
-
- Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. - PubMed
-
- Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. - PubMed
-
- Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

