In vivo overexpression of tissue-nonspecific alkaline phosphatase increases skeletal mineralization and affects the phosphorylation status of osteopontin
- PMID: 23427088
- PMCID: PMC3688694
- DOI: 10.1002/jbmr.1901
In vivo overexpression of tissue-nonspecific alkaline phosphatase increases skeletal mineralization and affects the phosphorylation status of osteopontin
Abstract
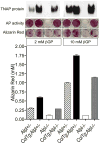
Functional ablation of tissue-nonspecific alkaline phosphatase (TNAP) (Alpl⁻/⁻ mice) leads to hypophosphatasia, characterized by rickets/osteomalacia attributable to elevated levels of extracellular inorganic pyrophosphate, a potent mineralization inhibitor. Osteopontin (OPN) is also elevated in the plasma and skeleton of Alpl⁻/⁻ mice. Phosphorylated OPN is known to inhibit mineralization, however, the phosphorylation status of the increased OPN found in Alpl⁻/⁻ mice is unknown. Here, we generated a transgenic mouse line expressing human TNAP under control of an osteoblast-specific Col1a1 promoter (Col1a1-Tnap). The transgene is expressed in osteoblasts, periosteum, and cortical bones, and plasma levels of TNAP in mice expressing Col1a1-Tnap are 10 to 20 times higher than those of wild-type mice. The Col1a1-Tnap animals are healthy and exhibit increased bone mineralization by micro-computed tomography (µCT) analysis. Crossbreeding of Col1a1-Tnap transgenic mice to Alpl⁻/⁻ mice rescues the lethal hypophosphatasia phenotype characteristic of this disease model. Osteoblasts from [Col1a1-Tnap] mice mineralize better than nontransgenic controls and osteoblasts from [Col1a1-Tnap⁺/⁻; Alpl⁻/⁻] mice are able to mineralize to the level of Alpl⁺/⁻ heterozygous osteoblasts, whereas Alpl⁻/⁻ osteoblasts show no mineralization. We found that the increased levels of OPN in bone tissue of Alpl⁻/⁻ mice are comprised of phosphorylated forms of OPN whereas wild-type (WT) and [Col1a1-Tnap⁺/⁻; Alpl⁻/⁻] mice had both phosphorylated and dephosphorylated forms of OPN. OPN from [Col1a1-Tnap] osteoblasts were more dephosphorylated than nontransgenic control cells. Titanium dioxide-liquid chromatography and tandem mass spectrometry analysis revealed that OPN peptides derived from Alpl⁻/⁻ bone and osteoblasts yielded a higher proportion of phosphorylated peptides than samples from WT mice, and at least two phosphopeptides, p(S¹⁷⁴FQVS¹⁷⁸DEQY¹⁸²PDAT¹⁸⁶DEDLT¹⁹¹)SHMK and FRIp(S²⁹⁹HELES³⁰⁴S³⁰⁵S³⁰⁶S³⁰⁷)EVN, with one nonlocalized site each, appear to be preferred sites of TNAP action on OPN. Our data suggest that the promineralization role of TNAP may be related not only to its accepted pyrophosphatase activity but also to its ability to modify the phosphorylation status of OPN.
Copyright © 2013 American Society for Bone and Mineral Research.
Conflict of interest statement
Conflict of interest: All authors report no conflicts of interest.
Figures





References
-
- Narisawa S, Hasegawa H, Watanabe K, Millán JL. Stage-specific expression of alkaline phosphatase during neural development in the mouse. Dev Dyn. 1994;201:227–35. - PubMed
-
- Narisawa S, Fröhlander N, Millán JL. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn. 1997;208:432–46. - PubMed
-
- Narisawa S, Wennberg S, Millán JL. Abnormal vitamin B6 metabolism in alkaline phosphatase knock-out mice causes multiple abnormalities, but not the impaired bone mineralization. J Pathol. 2001;193:125–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

