Tardbpl splicing rescues motor neuron and axonal development in a mutant tardbp zebrafish
- PMID: 23427147
- PMCID: PMC3658164
- DOI: 10.1093/hmg/ddt082
Tardbpl splicing rescues motor neuron and axonal development in a mutant tardbp zebrafish
Abstract
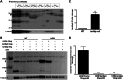
Mutations in the transactive response DNA binding protein-43 (TARDBP/TDP-43) gene, which regulates transcription and splicing, causes a familial form of amyotrophic lateral sclerosis (ALS). Here, we characterize and report the first tardbp mutation in zebrafish, which introduces a premature stop codon (Y220X), eliminating expression of the Tardbp protein. Another TARDBP ortholog, tardbpl, in zebrafish is shown to encode a Tardbp-like protein which is truncated compared with Tardbp itself and lacks part of the C-terminal glycine-rich domain (GRD). Here, we show that tardbp mutation leads to the generation of a novel tardbpl splice form (tardbpl-FL) capable of making a full-length Tardbp protein (Tardbpl-FL), which compensates for the loss of Tardbp. This finding provides a novel in vivo model to study TDP-43-mediated splicing regulation. Additionally, we show that elimination of both zebrafish TARDBP orthologs results in a severe motor phenotype with shortened motor axons, locomotion defects and death at around 10 days post fertilization. The Tardbp/Tardbpl knockout model generated in this study provides an excellent in vivo system to study the role of the functional loss of Tardbp and its involvement in ALS pathogenesis.
Figures




References
-
- Ferraiuolo L., Kirby J., Grierson A.J., Sendtner M., Shaw P.J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011;7:616–630. - PubMed
-
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

