Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking
- PMID: 23427155
- PMCID: PMC3624325
- DOI: 10.1128/JVI.02524-12
Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking
Abstract
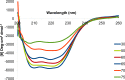
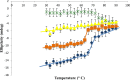
The importance of the phospholipase A2 domain located within the unique N terminus of the capsid viral protein VP1 (VP1u) in parvovirus infection has been reported. This study used computational methods to characterize the VP1 sequence for adeno-associated virus (AAV) serotypes 1 to 12 and circular dichroism and electron microscopy to monitor conformational changes in the AAV1 capsid induced by temperature and the pHs encountered during trafficking through the endocytic pathway. Circular dichroism was also used to monitor conformational changes in AAV6 capsids assembled from VP2 and VP3 or VP1, VP2, and VP3 at pH 7.5. VP1u was predicted (computationally) and confirmed (in solution) to be structurally ordered. This VP domain was observed to undergo a reversible pH-induced unfolding/refolding process, a loss/gain of α-helical structure, which did not disrupt the capsid integrity and is likely facilitated by its difference in isoelectric point compared to the other VP sequences assembling the capsid. This study is the first to physically document conformational changes in the VP1u region that likely facilitate its externalization from the capsid interior during infection and establishes the order of events in the escape of the AAV capsid from the endosome en route to the nucleus.
Figures





References
-
- Atchison RW, Casto BC, Hammon WM. 1965. Adenovirus-associated defective virus particles. Science 149:754–755 - PubMed
-
- Geoffroy M-C, Salvetti A. 2005. Helper functions required for wild type and recombinant adeno-associated virus growth. Curr. Gene Ther. 5:265–271 - PubMed
-
- Walz C, Deprez A, Dupressoir T, Dürst M, Rabreau M, Schlehofer JR. 1997. Interaction of human papillomavirus type 16 and adeno-associated virus type 2 co-infecting human cervical epithelium. J. Gen. Virol. 78(Part 6):1441–1452 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

