The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes
- PMID: 23427265
- PMCID: PMC3623635
- DOI: 10.1091/mbc.E12-11-0801
The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes
Abstract
The nexin-dynein regulatory complex (N-DRC) is proposed to coordinate dynein arm activity and interconnect doublet microtubules. Here we identify a conserved region in DRC4 critical for assembly of the N-DRC into the axoneme. At least 10 subunits associate with DRC4 to form a discrete complex distinct from other axonemal substructures. Transformation of drc4 mutants with epitope-tagged DRC4 rescues the motility defects and restores assembly of missing DRC subunits and associated inner-arm dyneins. Four new DRC subunits contain calcium-signaling motifs and/or AAA domains and are nearly ubiquitous in species with motile cilia. However, drc mutants are motile and maintain the 9 + 2 organization of the axoneme. To evaluate the function of the N-DRC, we analyzed ATP-induced reactivation of isolated axonemes. Rather than the reactivated bending observed with wild-type axonemes, ATP addition to drc-mutant axonemes resulted in splaying of doublets in the distal region, followed by oscillatory bending between pairs of doublets. Thus the N-DRC provides some but not all of the resistance to microtubule sliding and helps to maintain optimal alignment of doublets for productive flagellar motility. These findings provide new insights into the mechanisms that regulate motility and further highlight the importance of the proximal region of the axoneme in generating flagellar bending.
Figures

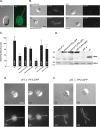
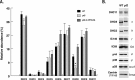



References
-
- Armbrust EV, et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science. 2004;306:79–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

