Single-subject fMRI mapping at 7 T of the representation of fingertips in S1: a comparison of event-related and phase-encoding designs
- PMID: 23427300
- PMCID: PMC3652218
- DOI: 10.1152/jn.00499.2012
Single-subject fMRI mapping at 7 T of the representation of fingertips in S1: a comparison of event-related and phase-encoding designs
Abstract
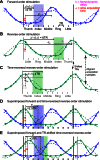
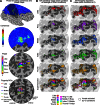
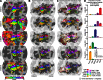
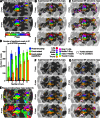
A desirable goal of functional MRI (fMRI), both clinically and for basic research, is to produce detailed maps of cortical function in individual subjects. Single-subject mapping of the somatotopic hand representation in the human primary somatosensory cortex (S1) has been performed using both phase-encoding and block/event-related designs. Here, we review the theoretical strengths and limits of each method and empirically compare high-resolution (1.5 mm isotropic) somatotopic maps obtained using fMRI at ultrahigh magnetic field (7 T) with phase-encoding and event-related designs in six subjects in response to vibrotactile stimulation of the five fingertips. Results show that the phase-encoding design is more efficient than the event-related design for mapping fingertip-specific responses and in particular allows us to describe a new additional somatotopic representation of fingertips on the precentral gyrus. However, with sufficient data, both designs yield very similar fingertip-specific maps in S1, which confirms that the assumption of local representational continuity underlying phase-encoding designs is largely valid at the level of the fingertips in S1. In addition, it is shown that the event-related design allows the mapping of overlapping cortical representations that are difficult to estimate using the phase-encoding design. The event-related data show a complex pattern of overlapping cortical representations for different fingertips within S1 and demonstrate that regions of S1 responding to several adjacent fingertips can incorrectly be identified as responding preferentially to one fingertip in the phase-encoding data.
Keywords: high-field MRI; high-resolution functional MRI; human; somatosensory cortex; tactile perception.
Figures


References
-
- Andersson JL, Jenkinson M, Smith SM. Non-Linear Registration aka Spatial Normalisation. 2007
-
- Baseler HA, Gouws A, Haak KV, Racey C, Crossland MD, Tufail A, Rubin GS, Cornelissen FW, Morland AB. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat Neurosci 14: 649–655, 2011 - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300, 1995
-
- Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93: 491–507, 2006
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

