Population-based study on use of chemotherapy in men with castration resistant prostate cancer
- PMID: 23427879
- PMCID: PMC3812701
- DOI: 10.3109/0284186X.2013.770164
Population-based study on use of chemotherapy in men with castration resistant prostate cancer
Abstract
Background: Chemotherapy prolongs life and relieves symptoms in men with castration resistant prostate cancer (CRPC). There is limited information on a population level on the use of chemotherapy for CRPC.
Material and methods: To assess the use of chemotherapy in men with CRPC we conducted a register-based nationwide population-based study in Prostate Cancer data Base Sweden (PCBaSe) and a nationwide in-patient drug register (SALT database) between May 2009 and December 2010. We assumed that men who died of prostate cancer (PCa) underwent a period of CRPC before they died.
Results: Among the 2677 men who died from PCa during the study inclusion period, 556 (21%) had received chemotherapy (intravenous or per oral) detectable within the observation period in SALT database. Specifically, 239 (61%) of men < 70 years had received chemotherapy, 246 (30%) of men between 70 and 79 years and 71 (5%) men older than 80 years. The majority of men 465/556 (84%) had received a docetaxel-containing regimen. Among chemotherapy treated men, 283/556 (51%) received their last dose of chemotherapy during the last six months prior to death. Treatment with chemotherapy was more common among men with little comorbidity and high educational level, as well as in men who had received curatively intended primary treatment.
Conclusion: A majority of men younger than 70 years with CRPC were treated with chemotherapy in contrast to men between 70 and 79 years of whom half as many received chemotherapy. Chemotherapy treatment was often administered shortly prior to death. The low uptake of chemotherapy in older men with CRPC may be caused by concerns about tolerability of treatment, as well as treatment decisions based on chronological age rather than global health status.
Figures
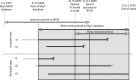

Comment in
-
What is the appropriate use of palliative docetaxel in castration-resistant prostate cancer?Acta Oncol. 2013 Nov;52(8):1589-92. doi: 10.3109/0284186X.2013.821206. Acta Oncol. 2013. PMID: 24102178 No abstract available.
References
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. - PubMed
-
- Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–83. - PubMed
-
- Fossa SD, Jacobsen AB, Ginman C, Jacobsen IN, Overn S, Iversen JR, et al. Weekly docetaxel and prednisolone versus prednisolone alone in androgen-independent prostate cancer: A randomized phase II study. Eur Urol. 2007;52:1691–8. - PubMed
-
- Berthold DR, Pond GR, de Wit R, Eisenberger M, Tannock IF. Survival and PSA response of patients in the TAX 327 study who crossed over to receive docetaxel after mitoxantrone or vice versa. Ann Oncol. 2008;19:1749–53. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
