The zinc-finger domain was essential for porcine reproductive and respiratory syndrome virus nonstructural protein-1α to inhibit the production of interferon-β
- PMID: 23428052
- PMCID: PMC3665301
- DOI: 10.1089/jir.2012.0100
The zinc-finger domain was essential for porcine reproductive and respiratory syndrome virus nonstructural protein-1α to inhibit the production of interferon-β
Abstract
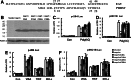
Porcine reproductive and respiratory syndrome virus (PRRSV) has caused one of the most economically devastating and pandemic diseases of swine. Previous studies have documented that PRRSV nonstructural protein-1α (nsp1α) was an interferon antagonist, but the mechanism by which nsp1α inhibited the interferon (IFN)-β production was unclear. Here, by site-directed mutagenesis of the predicted zinc-coordinating residues of the zinc-finger (ZF) domain of nsp1α or by deletion of the ZF domain of nsp1α, we explored whether the ZF domain was required for nsp1α to disrupt the IFN-β production. The results showed that both mutagenesis of the predicted zinc-coordinating residues of the ZF domain and deletion of the ZF domain made nsp1α lose its interferon antagonism activity. In conclusion, our present work indicated that the ZF domain of nsp1α was necessary for nsp1α to inhibit the IFN-β induction.
Figures
Similar articles
-
Amino acid at position 176 was essential for porcine reproductive and respiratory syndrome virus (PRRSV) non-structural protein 1α (nsp1α) as an inhibitor to the induction of IFN-β.Cell Immunol. 2012 Dec;280(2):125-31. doi: 10.1016/j.cellimm.2012.10.003. Epub 2012 Oct 29. Cell Immunol. 2012. PMID: 23399837
-
Nonstructural protein 1α subunit-based inhibition of NF-κB activation and suppression of interferon-β production by porcine reproductive and respiratory syndrome virus.Virology. 2010 Nov 25;407(2):268-80. doi: 10.1016/j.virol.2010.08.025. Epub 2010 Sep 17. Virology. 2010. PMID: 20850164
-
Nuclear export signal of PRRSV NSP1α is necessary for type I IFN inhibition.Virology. 2016 Dec;499:278-287. doi: 10.1016/j.virol.2016.07.008. Epub 2016 Oct 6. Virology. 2016. PMID: 27718457
-
Mechanisms of suppression of interferon production by porcine reproductive and respiratory syndrome virus.Acta Virol. 2012;56(1):3-9. doi: 10.4149/av_2012_01_3. Acta Virol. 2012. PMID: 22404603 Review.
-
Modulation of innate immune signaling by nonstructural protein 1 (nsp1) in the family Arteriviridae.Virus Res. 2014 Dec 19;194:100-9. doi: 10.1016/j.virusres.2014.09.007. Epub 2014 Sep 28. Virus Res. 2014. PMID: 25262851 Free PMC article. Review.
Cited by
-
The Amino-Terminal Region of Hepatitis E Virus ORF1 Containing a Methyltransferase (Met) and a Papain-Like Cysteine Protease (PCP) Domain Counteracts Type I Interferon Response.Viruses. 2018 Dec 18;10(12):726. doi: 10.3390/v10120726. Viruses. 2018. PMID: 30567349 Free PMC article.
-
Karyopherin Alpha 6 Is Required for Replication of Porcine Reproductive and Respiratory Syndrome Virus and Zika Virus.J Virol. 2018 Apr 13;92(9):e00072-18. doi: 10.1128/JVI.00072-18. Print 2018 May 1. J Virol. 2018. PMID: 29444946 Free PMC article.
-
Porcine Reproductive and Respiratory Syndrome Virus nsp1α Inhibits NF-κB Activation by Targeting the Linear Ubiquitin Chain Assembly Complex.J Virol. 2017 Jan 18;91(3):e01911-16. doi: 10.1128/JVI.01911-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881655 Free PMC article.
-
Nsp1α of Porcine Reproductive and Respiratory Syndrome Virus Strain BB0907 Impairs the Function of Monocyte-Derived Dendritic Cells via the Release of Soluble CD83.J Virol. 2018 Jul 17;92(15):e00366-18. doi: 10.1128/JVI.00366-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29793955 Free PMC article.
-
Nonstructural protein 11 (nsp11) of porcine reproductive and respiratory syndrome virus (PRRSV) promotes PRRSV infection in MARC-145 cells.BMC Vet Res. 2016 Jun 6;12:90. doi: 10.1186/s12917-016-0717-5. BMC Vet Res. 2016. PMID: 27268206 Free PMC article.
References
-
- Buddaert W. Van Reeth K. Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV) Adv Exp Med Biol. 1998;440:461–467. - PubMed
-
- Cavanagh D. Nidovirales: a new order comprising coronaviridae and arteriviridae. Arch Virol. 1997;142:629–633. - PubMed
-
- Chen Z. Lawson S. Sun Z. Zhou X. Guan X. Christopher-Hennings J. Nelson EA. Fang Y. Identification of two auto-cleavage products of nonstructural protein 1 (nsp1) in porcine reproductive and respiratory syndrome virus infected cells: nsp1 function as interferon antagonist. Virology. 2010;398:87–97. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

