The endothelial glycocalyx in syndecan-1 deficient mice
- PMID: 23428342
- PMCID: PMC3627742
- DOI: 10.1016/j.mvr.2013.02.001
The endothelial glycocalyx in syndecan-1 deficient mice
Abstract
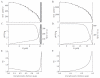
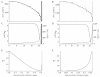
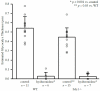
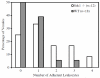
The existence of a hydrodynamically relevant endothelial glycocalyx has been established in capillaries, venules, and arterioles in vivo. The glycocalyx is thought to consist primarily of membrane-bound proteoglycans with glycosaminoglycan side-chains, membrane-bound glypicans, and adsorbed plasma proteins. The proteoglycans found on the luminal surface of endothelial cells are syndecans-1, -2, and -4, and glypican-1. The extent to which any of these proteins might serve to anchor the glycocalyx to the endothelium has not yet been determined. To test whether syndecan-1, in particular, is an essential anchoring protein, we performed experiments to determine the hydrodynamically relevant glycocalyx thickness in syndecan-1 deficient (Sdc1(-/-)) mice. Micro-particle image velocimetry data were collected using a previously described method. Microviscometric analysis of these data consistently revealed the existence of a hydrodynamically relevant endothelial glycocalyx in Sdc1(-/-) mice in vivo. The mean glycocalyx thickness found in Sdc1(-/-) mice was 0.45±0.10 μm (N=15), as compared with 0.54±0.12 μm (N=11) in wild-type (WT) mice (p=0.03). The slightly thinner glycocalyx observed in Sdc1(-/-) mice relative to WT mice may be due to the absence of syndecan-1. These findings show that healthy Sdc1(-/-) mice are able to synthesize and maintain a hydrodynamically relevant glycocalyx, which indicates that syndecan-1 is not an essential anchoring protein for the glycocalyx in Sdc1(-/-) mice. This may also be the case for WT mice; however, Sdc1(-/-) mice might adapt to the lack of syndecan-1 by increasing the expression of other proteoglycans. In any case, syndecan-1 does not appear to be a prerequisite for the existence of an endothelial glycocalyx.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat. Gen. 2000;25:329–332. - PubMed
-
- Alexopoulou AN, Multhaupt HAB, Couchman JR. Syndecans in wound healing, inflammation, and vascular biology. Int. J. Biochem. Cell Biol. 2007;39:505–528. - PubMed
-
- Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc. Res. 1973;5:384–394. - PubMed
-
- Barker AL, Konopatskaya O, Neal CR, Macpherson JV, Whatmore JL, Winlove PC, Unwin PR, Shore AC. Observation and characterisation of the glycocalyx of viable human endothelial cells using confocal laser scanning microscopy. Int. Phys. Chem. Chem. Phys. 2004;6:1006–1011.
-
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan-sulfate proteoglycans. Annu. Rev. Cell Biol. 1992;8:365–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

