The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases
- PMID: 23429354
- PMCID: PMC4553985
- DOI: 10.1097/MD.0b013e318288925c
The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases
Abstract
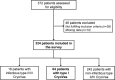
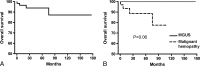
Type I cryoglobulinemia vasculitis (CryoVas) is considered a life-threatening condition; however, data on the characteristics and outcome are scarce. To analyze the presentation, prognosis, and efficacy and safety of treatments of type I CryoVas, we conducted a French nationwide survey that included 64 patients with type I CryoVas between January 1995 and July 2010: 28 patients with monoclonal gammopathy of unknown significance (MGUS) and 36 with hematologic malignancy.Type I monoclonal CryoVas was characterized by severe cutaneous involvement (necrosis and ulcers) in almost half the patients and high serum cryoglobulin levels, contrasting with a lower frequency of glomerulonephritis than expected. The 1-, 3-, 5-, and 10-year survival rates were 97%, 94%, 94%, and 87%, respectively. Compared to MGUS, type I CryoVas related to hematologic malignancy tended to be associated with a poorer prognosis. Therapeutic regimens based on alkylating agents, rituximab, thalidomide or lenalinomide, and bortezomib showed similar efficacy on vasculitis manifestations, with clinical response rates from 80% to 86%.Data from the CryoVas survey show that the prognosis of type I CryoVas does not seem to be as poor as previously suggested. Besides alkylating agents, the use of regimens based on rituximab, thalidomide or lenalinomide, and bortezomib are interesting alternative options, although the exact role of each strategy remains to be defined.
Figures

References
-
- Anonymous. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003; 121: 749– 757. - PubMed
-
- Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974; 57: 775– 788. - PubMed
-
- Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999; 42: 2204– 2212. - PubMed
-
- Calabrese C, Faiman B, Martin D, Reu F, Calabrese LH. Type 1 cryoglobulinemia: response to thalidomide and lenalidomide. J Clin Rheumatol. 2011; 17: 145– 147. - PubMed
-
- Cem Ar M, Soysal T, Hatemi G, Salihoglu A, Yazici H, Ulku B. Successful management of cryoglobulinemia-induced leukocytoclastic vasculitis with thalidomide in a patient with multiple myeloma. Ann Hematol. 2005; 84: 609– 613. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

