Trichomonas vaginalis contact-dependent cytolysis of epithelial cells
- PMID: 23429535
- PMCID: PMC3648012
- DOI: 10.1128/IAI.01244-12
Trichomonas vaginalis contact-dependent cytolysis of epithelial cells
Abstract
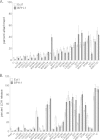
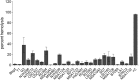
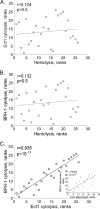
Trichomonas vaginalis is an extracellular protozoan parasite that binds to the epithelium of the human urogenital tract during infection. In this study, we examined the propensities of 26 T. vaginalis strains to bind to and lyse prostate (BPH-1) and ectocervical (Ect1) epithelium and to lyse red blood cells (RBCs). We found that only three of the strains had a statistically significant preference for either BPH-1 (MSA1103) or Ect1 (LA1 and MSA1123). Overall, we observed that levels of adherence are highly variable among strains, with a 12-fold range of adherence on Ect1 cells and a 45-fold range on BPH-1 cells. Cytolysis levels displayed even greater variability, from no detectable cytolysis to 80% or 90% cytolysis of Ect1 and BPH-1, respectively. Levels of adherence and cytolysis correlate for weakly adherent/cytolytic strains, and a threshold of attachment was found to be necessary to trigger cytolysis; however, this threshold can be reached without inducing cytolysis. Furthermore, cytolysis was completely blocked when we prevented attachment of the parasites to host cells while allowing soluble factors complete access. We demonstrate that hemolysis was a rare trait, with only 4 of the 26 strains capable of lysing >20% RBCs with a 1:30 parasite/RBC ratio. Hemolysis also did not correlate with adherence to or cytolysis of either male (BPH-1)- or female (Ect1)-derived epithelial cell lines. Our results reveal that despite a broad range of pathogenic properties among different T. vaginalis strains, all strains show strict contact-dependent cytolysis.
Figures



Similar articles
-
Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host∶parasite interactions.PLoS Pathog. 2013;9(7):e1003482. doi: 10.1371/journal.ppat.1003482. Epub 2013 Jul 11. PLoS Pathog. 2013. PMID: 23853596 Free PMC article.
-
A Novel Trichomonas vaginalis Surface Protein Modulates Parasite Attachment via Protein:Host Cell Proteoglycan Interaction.mBio. 2021 Feb 9;12(1):e03374-20. doi: 10.1128/mBio.03374-20. mBio. 2021. PMID: 33563826 Free PMC article.
-
A Novel Cadherin-like Protein Mediates Adherence to and Killing of Host Cells by the Parasite Trichomonas vaginalis.mBio. 2019 May 14;10(3):e00720-19. doi: 10.1128/mBio.00720-19. mBio. 2019. PMID: 31088924 Free PMC article.
-
[Research progress on the adhesion course of Trichomonas vaginalis].Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005 Feb 28;23(1):56-8. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005. PMID: 16042210 Review. Chinese. No abstract available.
-
The biology of Trichomonas vaginalis in the light of urogenital tract infection.Mol Biochem Parasitol. 2014 Dec;198(2):92-9. doi: 10.1016/j.molbiopara.2015.01.004. Epub 2015 Feb 9. Mol Biochem Parasitol. 2014. PMID: 25677793 Review.
Cited by
-
Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host∶parasite interactions.PLoS Pathog. 2013;9(7):e1003482. doi: 10.1371/journal.ppat.1003482. Epub 2013 Jul 11. PLoS Pathog. 2013. PMID: 23853596 Free PMC article.
-
Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):8179-84. doi: 10.1073/pnas.1321884111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843155 Free PMC article.
-
Neglected parasitic infections in the United States: trichomoniasis.Am J Trop Med Hyg. 2014 May;90(5):800-804. doi: 10.4269/ajtmh.13-0723. Am J Trop Med Hyg. 2014. PMID: 24808247 Free PMC article.
-
Dynamic secretome of Trichomonas vaginalis: Case study of β-amylases.Mol Cell Proteomics. 2018 Feb;17(2):304-320. doi: 10.1074/mcp.RA117.000434. Epub 2017 Dec 12. Mol Cell Proteomics. 2018. PMID: 29233912 Free PMC article.
-
Learning the language of pathogens.Elife. 2023 Jun 15;12:e89264. doi: 10.7554/eLife.89264. Elife. 2023. PMID: 37318983 Free PMC article.
References
-
- Rowley R, Toskin I, Ndowa F. 2012. Global incidence and prevalence of selected curable sexually transmitted infections—2008. 978 92 4 150383 9. WHO, Geneva, Switzerland
-
- Schwebke JR, Hook EW., III 2003. High rates of Trichomonas vaginalis among men attending a sexually transmitted diseases clinic: implications for screening and urethritis management. J. Infect. Dis. 188:465–468 - PubMed
-
- Sena AC, Miller WC, Hobbs MM, Schwebke JR, Leone PA, Swygard H, Atashili J, Cohen MS. 2007. Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment, and prevention. Clin. Infect. Dis. 44:13–22 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

