The absence of myocardial calcium-independent phospholipase A2γ results in impaired prostaglandin E2 production and decreased survival in mice with acute Trypanosoma cruzi infection
- PMID: 23429536
- PMCID: PMC3697611
- DOI: 10.1128/IAI.00497-12
The absence of myocardial calcium-independent phospholipase A2γ results in impaired prostaglandin E2 production and decreased survival in mice with acute Trypanosoma cruzi infection
Abstract
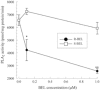
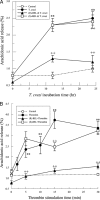
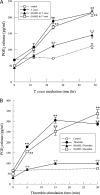
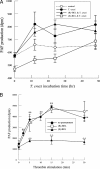
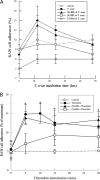
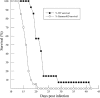
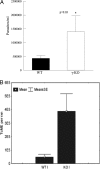
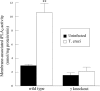
Cardiomyopathy is a serious complication of Chagas' disease, caused by the protozoan parasite Trypanosoma cruzi. The parasite often infects cardiac myocytes, causing the release of inflammatory mediators, including eicosanoids. A recent study from our laboratory demonstrated that calcium-independent phospholipase A2γ (iPLA2γ) accounts for the majority of PLA2 activity in rabbit ventricular myocytes and is responsible for arachidonic acid (AA) and prostaglandin E2 (PGE2) release. Thus, we hypothesized that cardiac iPLA2γ contributes to eicosanoid production in T. cruzi infection. Inhibition of the isoform iPLA2γ or iPLA2β, with the R or S enantiomer of bromoenol lactone (BEL), respectively, demonstrated that iPLA2γ is the predominant isoform in immortalized mouse cardiac myocytes (HL-1 cells). Stimulation of HL-1 cells with thrombin, a serine protease associated with microthrombus formation in Chagas' disease and a known activator of iPLA2, increased AA and PGE2 release, accompanied by platelet-activating factor (PAF) production. Similarly, T. cruzi infection resulted in increased AA and PGE2 release over time that was inhibited by pretreatment with (R)-BEL. Further, T. cruzi-infected iPLA2γ-knockout (KO) mice had lower survival rates and increased tissue parasitism compared to wild-type (WT) mice, suggesting that iPLA2γ-KO mice were more susceptible to infection than WT mice. A significant increase in iPLA2 activity was observed in WT mice following infection, whereas iPLA2γ-KO mice showed no alteration in cardiac iPLA2 activity and produced less PGE2. In summary, these studies demonstrate that T. cruzi infection activates cardiac myocyte iPLA2γ, resulting in increased AA and PGE2 release, mediators that may be essential for host survival during acute infection. Thus, these studies suggest that iPLA2γ plays a cardioprotective role during the acute stage of Chagas' disease.
Figures
Similar articles
-
Mice with Genetic Deletion of Group VIA Phospholipase A2β Exhibit Impaired Macrophage Function and Increased Parasite Load in Trypanosoma cruzi-Induced Myocarditis.Infect Immun. 2016 Mar 24;84(4):1137-1142. doi: 10.1128/IAI.01564-15. Print 2016 Apr. Infect Immun. 2016. PMID: 26857573 Free PMC article.
-
Cardioprotective actions of curcumin on the pathogenic NFAT/COX-2/prostaglandin E2 pathway induced during Trypanosoma cruzi infection.Phytomedicine. 2016 Nov 15;23(12):1392-1400. doi: 10.1016/j.phymed.2016.06.017. Epub 2016 Jun 29. Phytomedicine. 2016. PMID: 27765359
-
Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids.J Biol Chem. 2012 Apr 27;287(18):14880-95. doi: 10.1074/jbc.M111.336776. Epub 2012 Mar 2. J Biol Chem. 2012. PMID: 22389508 Free PMC article.
-
Bioactive lipids in Trypanosoma cruzi infection.Adv Parasitol. 2011;76:1-31. doi: 10.1016/B978-0-12-385895-5.00001-3. Adv Parasitol. 2011. PMID: 21884885 Free PMC article. Review.
-
Calcium-independent phospholipase A2γ (iPLA2γ) and its roles in cellular functions and diseases.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):861-868. doi: 10.1016/j.bbalip.2018.10.009. Epub 2018 Nov 1. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30391710 Review.
Cited by
-
Disease Tolerance and Pathogen Resistance Genes May Underlie Trypanosoma cruzi Persistence and Differential Progression to Chagas Disease Cardiomyopathy.Front Immunol. 2018 Dec 3;9:2791. doi: 10.3389/fimmu.2018.02791. eCollection 2018. Front Immunol. 2018. PMID: 30559742 Free PMC article.
-
Inflammatory and Pro-resolving Lipids in Trypanosomatid Infections: A Key to Understanding Parasite Control.Front Microbiol. 2018 Aug 21;9:1961. doi: 10.3389/fmicb.2018.01961. eCollection 2018. Front Microbiol. 2018. PMID: 30186271 Free PMC article. Review.
-
Lipid hijacking: a unifying theme in vector-borne diseases.Elife. 2020 Oct 29;9:e61675. doi: 10.7554/eLife.61675. Elife. 2020. PMID: 33118933 Free PMC article. Review.
-
Role of cyclooxygenase-2 in Trypanosoma cruzi survival in the early stages of parasite host-cell interaction.Mem Inst Oswaldo Cruz. 2015 Apr;110(2):181-91. doi: 10.1590/0074-02760140311. Mem Inst Oswaldo Cruz. 2015. PMID: 25946241 Free PMC article.
-
COX-2 rs20417 Polymorphism Is Associated with Stroke and White Matter Disease.J Stroke Cerebrovasc Dis. 2015 Aug;24(8):1817-22. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.018. Epub 2015 May 6. J Stroke Cerebrovasc Dis. 2015. PMID: 25957909 Free PMC article.
References
-
- WHO 2012. WHO factsheet for Chagas' disease. WHO, Geneva, Switzerland
-
- Rassi A, Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402 - PubMed
-
- Rossi MA, Tanowitz HB, Malvestio LM, Celes MR, Campos EC, Blefari V, Prado CM. 2010. Coronary microvascular disease in chronic Chagas cardiomyopathy including an overview on history, pathology, and other proposed pathogenic mechanisms. PLoS Negl. Trop. Dis. 4:e674.10.1371/journal.pntd.0000674 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

