Optical coherence tomography--current and future applications
- PMID: 23429598
- PMCID: PMC3758124
- DOI: 10.1097/ICU.0b013e32835f8bf8
Optical coherence tomography--current and future applications
Abstract
Purpose of review: Optical coherence tomography (OCT) has revolutionized the clinical practice of ophthalmology. It is a noninvasive imaging technique that provides high-resolution, cross-sectional images of the retina, retinal nerve fiber layer and the optic nerve head. This review discusses the present applications of the commercially available spectral-domain OCT (SD-OCT) systems in the diagnosis and management of retinal diseases, with particular emphasis on choroidal imaging. Future directions of OCT technology and their potential clinical uses are discussed.
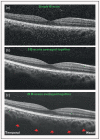
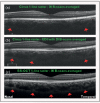
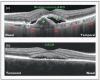
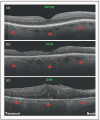
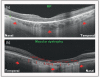
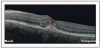
Recent findings: Analysis of the choroidal thickness in healthy eyes and disease states such as age-related macular degeneration, central serous chorioretinopathy, diabetic retinopathy and inherited retinal dystrophies has been successfully achieved using SD-OCT devices with software improvements. Future OCT innovations such as longer-wavelength OCT systems including the swept-source technology, along with Doppler OCT and en-face imaging, may improve the detection of subtle microstructural changes in chorioretinal diseases by improving imaging of the choroid.
Summary: Advances in OCT technology provide for better understanding of pathogenesis, improved monitoring of progression and assistance in quantifying response to treatment modalities in diseases of the posterior segment of the eye. Further improvements in both hardware and software technologies should further advance the clinician's ability to assess and manage chorioretinal diseases.
Figures


References
-
- de Boer JF, Cense B, Park BH, et al. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett. 2003;28:2067–2069. - PubMed
-
- Leitgeb R, Hitzenberger C, Fercher A. Performance of Fourier domain vs. time domain optical coherence tomography. Opt Express. 2003;11:889–894. - PubMed
-
- Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

