Molecular pathogenesis and mechanisms of thyroid cancer
- PMID: 23429735
- PMCID: PMC3791171
- DOI: 10.1038/nrc3431
Molecular pathogenesis and mechanisms of thyroid cancer
Abstract
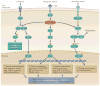
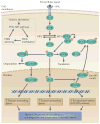
Thyroid cancer is a common endocrine malignancy. There has been exciting progress in understanding its molecular pathogenesis in recent years, as best exemplified by the elucidation of the fundamental role of several major signalling pathways and related molecular derangements. Central to these mechanisms are the genetic and epigenetic alterations in these pathways, such as mutation, gene copy-number gain and aberrant gene methylation. Many of these molecular alterations represent novel diagnostic and prognostic molecular markers and therapeutic targets for thyroid cancer, which provide unprecedented opportunities for further research and clinical development of novel treatment strategies for this cancer.
Conflict of interest statement
The author declares competing financial interests: see Web version for details.
Figures

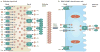
References
-
- Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Howlader N, et al. SEER Cancer Statistics Review 1975–2009 (Vintage 2009 Populations) National Cancer Institute. 2012 [online], http://seer.cancer.gov/csr/1975_2009_pops09.
-
- Tuttle RM, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274. - PubMed
-
- DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization Classification of Tumours. Pathology And Genetics Of Tumors Of Endocrine Organs. IARC Press; 2004. This book describes the most recent version of World Health Organization classification of thyroid tumours.
-
- Hofstra RM, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

