Response to methylphenidate by adult and pediatric patients with attention-deficit/hyperactivity disorder: the Spanish multicenter DIHANA study
- PMID: 23430373
- PMCID: PMC3573811
- DOI: 10.2147/NDT.S35836
Response to methylphenidate by adult and pediatric patients with attention-deficit/hyperactivity disorder: the Spanish multicenter DIHANA study
Abstract
Background: The purpose of this multicenter Spanish study was to evaluate the response to immediate-release methylphenidate by children and adults diagnosed with attention-deficit/hyperactivity disorder (ADHD), as well as to obtain information on current therapy patterns and safety characteristics.
Methods: This multicenter, observational, retrospective, noninterventional study included 730 patients aged 4-65 years with a diagnosis of ADHD. Information was obtained based on a review of medical records for the years 2002-2006 in sequential order.
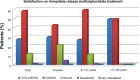
Results: The ADHD predominantly inattentive subtype affected 29.7% of patients, ADHD predominantly hyperactive-impulsive was found in 5.2%, and the combined subtype in 65.1%. Overall, a significant lower Clinical Global Impression (CGI) score and mean number of DSM-IV TR (Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision) symptoms by subtype were found after one year of treatment with immediate-release methylphenidate; CGI decreased from 4.51 to 1.69, symptoms of inattention from 7.90 to 4.34, symptoms of hyperactivity from 6.73 to 3.39, and combined subtype symptoms from 14.62 to 7.7. Satisfaction with immediate-release methylphenidate after one year was evaluated as "very satisfied" or "satisfied" by 86.90% of the sample; 25.75% of all patients reported at least one adverse effect. At the end of the study, 41.47% of all the patients treated with immediate-release methylphenidate were still receiving it, with a mean time of 3.80 years on therapy.
Conclusion: Good efficacy and safety results were found for immediate-release methylphenidate in patients with ADHD.
Keywords: ADHD; attention deficit hyperactivity disorder; methylphenidate; pharmacologic treatment; satisfaction.
Figures
References
-
- American Academy of Pediatrics Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105:1158–1170. - PubMed
-
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–818. - PubMed
-
- International Consensus Statement on ADHD Clin Child Fam Psychol Rev. 2002;5:89–111. - PubMed
-
- Kutcher S, Aman M, Brooks SJ, et al. International consensus statement on attention deficit hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol. 2004;14:11–28. - PubMed
-
- Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292:619–623. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources