Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals
- PMID: 23430606
- PMCID: PMC3640542
- DOI: 10.3390/toxins5020396
Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals
Abstract
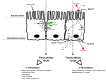
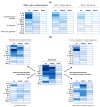
Mycotoxins are secondary metabolites of fungi that can cause serious health problems in animals, and may result in severe economic losses. Deleterious effects of these feed contaminants in animals are well documented, ranging from growth impairment, decreased resistance to pathogens, hepato- and nephrotoxicity to death. By contrast, data with regard to their impact on intestinal functions are more limited. However, intestinal cells are the first cells to be exposed to mycotoxins, and often at higher concentrations than other tissues. In addition, mycotoxins specifically target high protein turnover- and activated-cells, which are predominant in gut epithelium. Therefore, intestinal investigations have gained significant interest over the last decade, and some publications have demonstrated that mycotoxins are able to compromise several key functions of the gastrointestinal tract, including decreased surface area available for nutrient absorption, modulation of nutrient transporters, or loss of barrier function. In addition some mycotoxins facilitate persistence of intestinal pathogens and potentiate intestinal inflammation. By contrast, the effect of these fungal metabolites on the intestinal microbiota is largely unknown. This review focuses on mycotoxins which are of concern in terms of occurrence and toxicity, namely: aflatoxins, ochratoxin A and Fusarium toxins. Results from nearly 100 published experiments (in vitro, ex vivo and in vivo) were analyzed with a special attention to the doses used.
Figures

References
-
- Desjardins A., Maragos C., Norred W., Pestka J., Phillips T., Vardon P., Whitaker T., Wood G., van Egmond H. Mycotoxins:Risks in Plant, Animal, and Human System. Council for Agricultural Science and Technology; Ames, IA, USA: 2003.
-
- Bryden W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Tech. 2012;173:134–158. doi: 10.1016/j.anifeedsci.2011.12.014. - DOI
-
- Lorenzoni G. Poultry Diseases Influenced by Gastrointestinal Health: Traditional Treatments and Innovative Solutions. Nottingham University Press; Loughborough, UK: 2010. Poultry Diseases Influenced by Gastrointestinal Health: Traditional Treatments and Innovative Solutions; pp. 1–140.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

