A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings
- PMID: 23430708
- PMCID: PMC3715392
- DOI: 10.1093/ntr/ntt005
A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings
Abstract
Introduction: Identifying successful smoking treatment interventions and methods of delivery is critical given the smoking rates among HIV-positive populations and the medical implications of smoking in this population. This study compared the efficacy of 3 smoking cessation interventions provided in HIV clinical treatment settings.
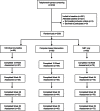
Methods: Following a baseline assessment, 209 HIV-positive smokers were randomly assigned to 1 of 3 conditions in a parallel group design. Treatment conditions were individual counseling plus nicotine replacement treatment (NRT), a computer-based Internet smoking treatment plus NRT, and self-help plus NRT. Smoking status was determined at follow-up assessments completed at 12, 24, 36, and 52 weeks following treatment initiation.
Results: Cessation rates ranged from 15% to 29%; however, no statistically significant differences in abstinence were found among the treatment conditions over time. Those employed, those who reported a greater desire to quit, or those with lower mood disturbance scores were more likely to achieve abstinence (p < .01). The number of cigarettes participants reported smoking in the 24hr prior to each assessment significantly declined over time (p < .001).
Conclusions: Although we found no differences in abstinence rates across groups, the results indicate that integration of smoking cessation interventions is feasible in HIV clinical treatment settings, and cessation results are promising. The overall abstinence rates we report are comparable to those found in similar treatment studies across multiple populations. Further research is warranted.
Figures

References
-
- Arnsten J. H., Li X., Mizuno Y., Knowlton A. R., Gourevitch M. N., Handley K, … for the INSPIRE Study Team (2007). Factors associated with antiretroviral therapy adherence and medication errors among HIV-infected injection drug users. Journal of Acquired Immune Deficiency Syndromes, 46(2), S64–S71.10.1097/QAI.0b013e31815767d6 - PubMed
-
- Barclay T. R., Hinkin C. H., Castellon S. A., Mason K. I., Reinhard M. J., Marion S. D, … Durvasula R. S. (2007). Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology, 26, 40–49.10.1037/0278-6133.26.1.40 - PMC - PubMed
-
- Boulter A. W., Soltanpoor N., Swan A. V., Birnbaum W., Johnson N. W., Teo C. G. (1996). Risk factors associated with Epstein-Barr virus replication in oral epithelial cells of HIV-infected individuals. AIDS, 10(9), 935–940.10.1097/00002030-199610090-00002 - PubMed
-
- Burkhalter J. E., Springer C. M., Chhabra R., Ostroff J. S., Rapkin B. D. (2005). Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine & Tobacco Research, 7(4), 511–522.10.1080/14622200500186064 - PubMed
-
- Burns D. N., Hillman D., Neaton J. D., Sherer R., Mitchell T., Capps L, … Gordin F. M. (1996). Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. Journal of Acquired Immune Deficiency Syndrome and Human Retrovirology, 13(4), 374–383. 10.1097/ 00042560-199612010-00012 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

