Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes
- PMID: 23430739
- PMCID: PMC3617250
- DOI: 10.1074/jbc.C112.444562
Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes
Abstract
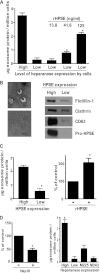
Emerging evidence indicates that exosomes play a key role in tumor-host cross-talk and that exosome secretion, composition, and functional capacity are altered as tumors progress to an aggressive phenotype. However, little is known regarding the mechanisms that regulate these changes. Heparanase is an enzyme whose expression is up-regulated as tumors become more aggressive and is associated with enhanced tumor growth, angiogenesis, and metastasis. We have discovered that in human cancer cells (myeloma, lymphoblastoid, and breast cancer), when expression of heparanase is enhanced or when tumor cells are exposed to exogenous heparanase, exosome secretion is dramatically increased. Heparanase enzyme activity is required for robust enhancement of exosome secretion because enzymatically inactive forms of heparanase, even when present in high amounts, do not dramatically increase exosome secretion. Heparanase also impacts exosome protein cargo as reflected by higher levels of syndecan-1, VEGF, and hepatocyte growth factor in exosomes secreted by heparanase-high expressing cells as compared with heparanase-low expressing cells. In functional assays, exosomes from heparanase-high cells stimulated spreading of tumor cells on fibronectin and invasion of endothelial cells through extracellular matrix better than did exosomes secreted by heparanase-low cells. These studies reveal that heparanase helps drive exosome secretion, alters exosome composition, and facilitates production of exosomes that impact both tumor and host cell behavior, thereby promoting tumor progression.
Figures

References
-
- Simons M., Raposo G. (2009) Exosomes–vesicular carriers for intercellular communication. Curr. Opin Cell Biol. 21, 575–581 - PubMed
-
- Théry C., Amigorena S., Raposo G., Clayton A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. in Current Protocols in Cell Biology, pp. 3.22.21–23.22.29, John Wiley & Sons, Inc., New York - PubMed
-
- Lee T. H., D'Asti E., Magnus N., Al-Nedawi K., Meehan B., Rak J. (2011) Microvesicles as mediators of intercellular communication in cancer–the emerging science of cellular 'debris'. Semin. Immunopathol. 33, 455–467 - PubMed
-
- Record M., Subra C., Silvente-Poirot S., Poirot M. (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 81, 1171–1182 - PubMed
-
- Hood J. L., San R. S., Wickline S. A. (2011) Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

