Three-dimensional structure of Saccharomyces invertase: role of a non-catalytic domain in oligomerization and substrate specificity
- PMID: 23430743
- PMCID: PMC3617277
- DOI: 10.1074/jbc.M112.446435
Three-dimensional structure of Saccharomyces invertase: role of a non-catalytic domain in oligomerization and substrate specificity
Abstract
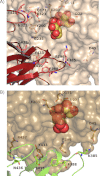
Invertase is an enzyme that is widely distributed among plants and microorganisms and that catalyzes the hydrolysis of the disaccharide sucrose into glucose and fructose. Despite the important physiological role of Saccharomyces invertase (SInv) and the historical relevance of this enzyme as a model in early biochemical studies, its structure had not yet been solved. We report here the crystal structure of recombinant SInv at 3.3 Å resolution showing that the enzyme folds into the catalytic β-propeller and β-sandwich domains characteristic of GH32 enzymes. However, SInv displays an unusual quaternary structure. Monomers associate in two different kinds of dimers, which are in turn assembled into an octamer, best described as a tetramer of dimers. Dimerization plays a determinant role in substrate specificity because this assembly sets steric constraints that limit the access to the active site of oligosaccharides of more than four units. Comparative analysis of GH32 enzymes showed that formation of the SInv octamer occurs through a β-sheet extension that seems unique to this enzyme. Interaction between dimers is determined by a short amino acid sequence at the beginning of the β-sandwich domain. Our results highlight the role of the non-catalytic domain in fine-tuning substrate specificity and thus supplement our knowledge of the activity of this important family of enzymes. In turn, this gives a deeper insight into the structural features that rule modularity and protein-carbohydrate recognition.
Figures






References
-
- Barnett J. A. (2003) Beginnings of microbiology and biochemistry: the contribution of yeast research. Microbiology 149, 557–567 - PubMed
-
- Michaelis L., Menten M. L. (1913) The Kinetics of Invertase Action. Biochem. Z. 49, 333–369
-
- de la Fuente G., Sols A. (1962) Transport of sugars in yeasts. II. Mechanisms of utilization of disaccharides and related glycosides. Biochim. Biophys. Acta 56, 49–62 - PubMed
-
- Gascón S., Lampen J. O. (1968) Purification of the internal invertase of yeast. J. Biol. Chem. 243, 1567–1572 - PubMed
-
- Gascón S., Neumann N. P., Lampen J. O. (1968) Comparative study of the properties of the purified internal and external invertases from yeast. J. Biol. Chem. 243, 1573–1577 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

