Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release
- PMID: 23430963
- PMCID: PMC3576869
- DOI: 10.3389/fimmu.2013.00038
Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release
Abstract
In response to inflammation, neutrophils deiminate histones and externalize chromatin. Neutrophil extracellular traps (NETs) are an innate immune defense mechanism, yet NETs also may aggravate chronic inflammatory and autoimmune disorders. Activation of peptidylarginine deiminase 4 (PAD4) is associated with NET release (NETosis) but the precise mechanisms of PAD4 regulation are unknown. We observed that, in human neutrophils, calcium ionophore induced histone deimination, whereas phorbol myristate acetate (PMA), an activator of protein kinase C (PKC), suppressed ionophore-induced deimination. Conversely, low doses of chelerythrine and sanguinarine, two inhibitors of PKC, reversed PMA inhibition and enhanced ionophore-stimulated deimination. In addition, a peptide inhibitor of PKCα superinduced ionophore activation of PAD4, thus identifying PKCα as the PMA-induced inhibitor of PAD4. At higher doses, chelerythrine, sanguinarine, and structurally unrelated PKC inhibitors blocked histone deimination, suggesting that a different PKC isoform activates histone deimination. We identify PKCζ as activator of PAD4 because a specific peptide inhibitor of this PKC isoform suppressed histone deimination. Confocal microscopy confirmed that, in the presence of PMA, NETosis proceeds without detectable histone deimination, and that ionophore cooperates with PMA to induce more extensive NET release. Broad inhibition of PKC by chelerythrine or specific inhibition of PKCζ suppressed NETosis. Our observations thus reveal an intricate antagonism between PKC isoforms in the regulation of histone deimination, identify a dominant role for PKCα in the repression of histone deimination, and assign essential functions to PKCζ in the activation of PAD4 and the execution of NETosis. The precise balance between opposing PKC isoforms in the regulation of NETosis affirms the idea that NET release underlies specific and vitally important evolutionary selection pressures.
Keywords: NETosis; PAD4; deimination; inflammation; protein kinase C.
Figures
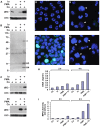


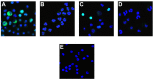
References
-
- Bertram A., Zhang H., von Vietinghoff S., de Pablo C., Haller H., Shushakova N., et al. (2012). Protein kinase C-t is required for murine neutrophil recruitment and adhesion strengthening under flow. J. Immunol. 188 4043–4051 - PubMed
-
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., et al. (2004). Neutrophil extracellular traps kill bacteria. Science 303 1532–1535 - PubMed
-
- Cuthbert G. L., Daujat S., Snowden A. W., Erdjument-Bromage H., Hagiwara T., Yamada M., et al. (2004). Histone deimination antagonizes arginine methylation. Cell 118 545–553 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

