DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo
- PMID: 23431033
- PMCID: PMC3627197
- DOI: 10.1093/infdis/jit047
DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo
Abstract
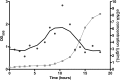
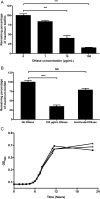
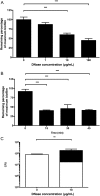
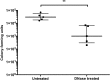
Bacterial vaginosis is a highly prevalent and poorly understood polymicrobial disorder of the vaginal microbiota, with significant adverse sequelae. Gardnerella vaginalis predominates in bacterial vaginosis. Biofilms of G. vaginalis are present in human infections and are implicated in persistent disease, treatment failure, and transmission. Here we demonstrate that G. vaginalis biofilms contain extracellular DNA, which is essential to their structural integrity. Enzymatic disruption of this DNA specifically inhibits biofilms, acting on both newly forming and established biofilms. DNase liberates bacteria from the biofilm to supernatant fractions and potentiates the activity of metronidazole, an antimicrobial agent used in the treatment of bacterial vaginosis. Using a new murine vaginal colonization model for G. vaginalis, we demonstrate >10-fold inhibition of G. vaginalis colonization by DNase. We conclude that DNase merits investigation as a potential nonantibiotic adjunct to existing bacterial vaginosis therapies in order to decrease the risk of chronic infection, recurrence, and associated morbidities.
Figures


Comment in
-
Vaginal biofilms and bacterial vaginosis: of mice and women.J Infect Dis. 2013 May 15;207(10):1481-3. doi: 10.1093/infdis/jit050. Epub 2013 Feb 19. J Infect Dis. 2013. PMID: 23431034 No abstract available.
-
Infection: treating G. vaginalis biofilms with DNase--a new strategy.Nat Rev Urol. 2013 Apr;10(4):188. doi: 10.1038/nrurol.2013.48. Epub 2013 Mar 12. Nat Rev Urol. 2013. PMID: 23478536 No abstract available.
References
-
- Schwebke JR. Gynecologic consequences of bacterial vaginosis. Obstet Gynecol Clin N Am. 2003;30:685–94. - PubMed
-
- Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

