Clinically meaningful treatment responses after switching to galantamine and with addition of memantine in patients with Alzheimer's disease receiving donepezil
- PMID: 23431041
- PMCID: PMC3575212
- DOI: 10.2147/NDT.S40682
Clinically meaningful treatment responses after switching to galantamine and with addition of memantine in patients with Alzheimer's disease receiving donepezil
Abstract
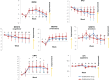
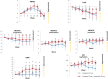
Clinical trials have shown the benefits of acetylcholinesterase inhibitors, such as donepezil and galantamine, and an N-methyl-D-aspartate receptor antagonist, memantine, in patients with Alzheimer's disease (AD). However, little is known regarding the effects of switching from donepezil 5 mg/day to galantamine 16 or 24 mg/day, or regarding the effects of adding memantine to established therapy compared with increasing the dose of donepezil. This report discusses two studies conducted to evaluate treatment with galantamine and memantine with respect to cognitive benefits and caregiver evaluations in patients with AD receiving donepezil 5 mg/day for more than 6 months. Patients with mild or moderate AD (scores 10-22 on the Mini-Mental State Examination) were enrolled in the Galantamine Switch study and switched to galantamine (maximum doses 16 mg versus 24 mg). Patients with moderate to severe AD (Mini-Mental State Examination scores 3-14) were enrolled in the Donepezil Increase versus Additional Memantine study and either had their donepezil dose increased to 10 mg/day or memantine 20 mg/day added to their existing donepezil dose. Patients received the study treatment for 28 weeks and their Disability Assessment for Dementia, Mental Function Impairment Scale, Cohen-Mansfield Agitation Inventory, and Neuropsychiatric Inventory scores were assessed with assistance from their caregivers. For the Galantamine Switch study after 8 weeks, agitation evaluated by the Cohen-Mansfield Agitation Inventory improved in both the 16 mg and 24 mg groups compared with baseline. However, there were no significant differences between the two galantamine groups. Agitation was also less in patients in the additional memantine group than in the donepezil increase group. In summary, switching to galantamine from donepezil and addition of memantine in patients with AD receiving donepezil were both safe and meaningful treatment options, and particularly efficacious for suppression of agitation.
Keywords: Alzheimer’s disease; acetylcholinesterase inhibitors; agitation; donepezil; galantamine; memantine.
Figures

References
-
- Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc. 1996;44(9):1078–1081. - PubMed
-
- Lopez OL, Becker JT, Sweet RA, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15(3):346–353. - PubMed
-
- Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54(12):2261–2268. - PubMed
-
- Winblad B. Maintaining functional and behavioral abilities in Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15( Suppl 1):S34–S40. - PubMed
-
- Samochocki M, Hoffe A, Fehrenbacher A, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305(3):1024–1036. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

