Factors associated with the suppressiveness of sugarcane soils to plant-parasitic nematodes
- PMID: 23431051
- PMCID: PMC3547352
Factors associated with the suppressiveness of sugarcane soils to plant-parasitic nematodes
Abstract
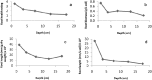
Observations in three Australian sugarcane fields suggested that the soil just under the trash blanket (the covering of crop residue that remains on the soil surface after crops are harvested) was suppressive to plant-parasitic nematodes. Roots were concentrated in this upper layer of soil but plant-parasitic nematode populations were relatively low and roots showed few signs of nematode damage. Root biomass was much lower 15 cm further down the soil profile, where root health was poor and populations of plant-parasitic nematodes were 3-5 times higher than near the soil surface. A bioassay in which Radopholus similis (a nematode that does not occur in sugarcane soils) was inoculated into heat-sterilized and untreated soils, confirmed that biological factors were limiting nematode populations in some of the soils, with soil from 0-2 cm much more suppressive than soil from 15-17 cm. Surface soil from one site was highly suppressive, as only 16% of R. similis recoverable from heated soil were retrieved from this soil after 8 days. Numerous soil chemical, biochemical, and biological properties were measured, and non-linear regression analysis identified two major groups of factors that were significantly associated with suppressiveness. One group reflected the amount of organic matter in soil (total C, total N, and labile C) and the other was associated with the size of the free-living nematode community (total numbers of free-living nematodes, and numbers of plant associates, bacterial feeders, fungal feeders, and carnivores). These results suggested that suppressiveness was biologically mediated and was sustained by C inputs from crop residues and roots. Since nematode-trapping fungi in the test soils could not be quantified using traditional dilution plating methods, their possible role as suppressive agents was assessed by generating TRFLP profiles with Orbiliales-specific primers, and by sequencing cloned PCR products. Although the molecular data were obtained from a limited number of samples, the level of suppression was significantly correlated to the number of Orbiliales clone groups and was also related to the number of Orbiliales species and TRFs, suggesting that this group of fungi may have been one of the suppressive factors operating in the test soils.
Keywords: Orbiliales; TRFLP; biological control; clone library; mulch; nematode community analysis; nematode-trapping fungi; organic matter-mediated suppression; predatory nematodes; sugarcane; suppressive soil.
Figures


References
-
- Ahrén D, Ursing BM, Tunlid A. Phylogeny of nematode trapping fungi based on 18S rDNA sequences. FEMS Microbiology Letters. 1998;158:179–184. - PubMed
-
- Akhtar M, Malik A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: a review. Bioresource Technology. 2000;74:35–47.
-
- Anderson IC, Campbell CD, Prosser JI. Potential bias of fungal 18s rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environmental Microbiology. 2003;5:36–47. - PubMed
-
- Benítez M- S, Tustas FB, Rotenberg D, Kleinhenz MD, Cardina J, Stinner D, Miller SA, McSpadden-Gardener BB. Multiple statistical approaches of community fingerprint data reveal bacterial populations associated with general disease suppression arising from the application of different organic field management strategies. Soil Biology and Biochemistry. 2007;39:2289–2301.
LinkOut - more resources
Full Text Sources
