The Hippo pathway: regulators and regulations
- PMID: 23431053
- PMCID: PMC3589553
- DOI: 10.1101/gad.210773.112
The Hippo pathway: regulators and regulations
Abstract
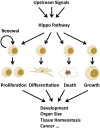
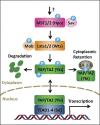
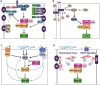
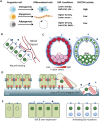
Control of cell number is crucial in animal development and tissue homeostasis, and its dysregulation may result in tumor formation or organ degeneration. The Hippo pathway in both Drosophila and mammals regulates cell number by modulating cell proliferation, cell death, and cell differentiation. Recently, numerous upstream components involved in the Hippo pathway have been identified, such as cell polarity, mechanotransduction, and G-protein-coupled receptor (GPCR) signaling. Actin cytoskeleton or cellular tension appears to be the master mediator that integrates and transmits upstream signals to the core Hippo signaling cascade. Here, we review regulatory mechanisms of the Hippo pathway and discuss potential implications involved in different physiological and pathological conditions.
Figures
References
-
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S 2012. Role of TAZ as mediator of Wnt signaling. Cell 151: 1443–1456 - PubMed
-
- Balazs L, Okolicany J, Ferrebee M, Tolley B, Tigyi G 2001. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo. Am J Physiol Regul Integr Comp Physiol 280: R466–R472 - PubMed
-
- Bao Y, Nakagawa K, Yang Z, Ikeda M, Withanage K, Ishigami-Yuasa M, Okuno Y, Hata S, Nishina H, Hata Y 2011. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem 150: 199–208 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
