Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel
- PMID: 23431132
- PMCID: PMC3593860
- DOI: 10.1073/pnas.1301889110
Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel
Abstract
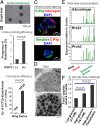
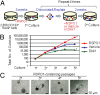
The study of hematopoietic colony-forming units using semisolid culture media has greatly advanced the knowledge of hematopoiesis. Here we report that similar methods can be used to study pancreatic colony-forming units. We have developed two pancreatic colony assays that enable quantitative and functional analyses of progenitor-like cells isolated from dissociated adult (2-4 mo old) murine pancreas. We find that a methylcellulose-based semisolid medium containing Matrigel allows growth of duct-like "Ring/Dense" colonies from a rare (∼1%) population of total pancreatic single cells. With the addition of roof plate-specific spondin 1, a wingless-int agonist, Ring/Dense colony-forming cells can be expanded more than 100,000-fold when serially dissociated and replated in the presence of Matrigel. When cells grown in Matrigel are then transferred to a Matrigel-free semisolid medium with a unique laminin-based hydrogel, some cells grow and differentiate into another type of colony, which we name "Endocrine/Acinar." These Endocrine/Acinar colonies are comprised mostly of endocrine- and acinar-like cells, as ascertained by RNA expression analysis, immunohistochemistry, and electron microscopy. Most Endocrine/Acinar colonies contain beta-like cells that secrete insulin/C-peptide in response to D-glucose and theophylline. These results demonstrate robust self-renewal and differentiation of adult Ring/Dense colony-forming units in vitro and suggest an approach to producing beta-like cells for cell replacement of type 1 diabetes. The methods described, which include microfluidic expression analysis of single cells and colonies, should also advance study of pancreas development and pancreatic progenitor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Colony-forming progenitor cells in the postnatal mouse liver and pancreas give rise to morphologically distinct insulin-expressing colonies in 3D cultures.Rev Diabet Stud. 2014 Spring;11(1):35-50. doi: 10.1900/RDS.2014.11.35. Epub 2014 May 10. Rev Diabet Stud. 2014. PMID: 25148366 Free PMC article.
-
Cells with surface expression of CD133highCD71low are enriched for tripotent colony-forming progenitor cells in the adult murine pancreas.Stem Cell Res. 2016 Jan;16(1):40-53. doi: 10.1016/j.scr.2015.11.015. Epub 2015 Nov 30. Stem Cell Res. 2016. PMID: 26691820 Free PMC article.
-
Adult Murine Pancreatic Progenitors Require Epidermal Growth Factor and Nicotinamide for Self-Renewal and Differentiation in a Serum- and Conditioned Medium-Free Culture.Stem Cells Dev. 2017 Apr 15;26(8):599-607. doi: 10.1089/scd.2016.0328. Epub 2017 Jan 17. Stem Cells Dev. 2017. PMID: 28095743 Free PMC article.
-
Gene regulatory networks governing pancreas development.Dev Cell. 2013 Apr 15;25(1):5-13. doi: 10.1016/j.devcel.2013.03.016. Dev Cell. 2013. PMID: 23597482 Free PMC article. Review.
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
Cited by
-
Organoids from the Human Fetal and Adult Pancreas.Curr Diab Rep. 2019 Dec 11;19(12):160. doi: 10.1007/s11892-019-1261-z. Curr Diab Rep. 2019. PMID: 31828551 Free PMC article. Review.
-
miR-29a contributes to breast cancer cells epithelial-mesenchymal transition, migration, and invasion via down-regulating histone H4K20 trimethylation through directly targeting SUV420H2.Cell Death Dis. 2019 Feb 21;10(3):176. doi: 10.1038/s41419-019-1437-0. Cell Death Dis. 2019. PMID: 30792382 Free PMC article.
-
Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis.EMBO J. 2013 Oct 16;32(20):2708-21. doi: 10.1038/emboj.2013.204. Epub 2013 Sep 17. EMBO J. 2013. PMID: 24045232 Free PMC article.
-
Heterogeneity of SOX9 and HNF1β in Pancreatic Ducts Is Dynamic.Stem Cell Reports. 2018 Mar 13;10(3):725-738. doi: 10.1016/j.stemcr.2018.01.028. Epub 2018 Mar 1. Stem Cell Reports. 2018. PMID: 29478894 Free PMC article.
-
Activation of ductal progenitor-like cells from adult human pancreas requires extracellular matrix protein signaling.iScience. 2024 Feb 15;27(3):109237. doi: 10.1016/j.isci.2024.109237. eCollection 2024 Mar 15. iScience. 2024. PMID: 38433896 Free PMC article.
References
-
- Gao R, Ustinov J, Korsgren O, Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005;48(11):2296–2304. - PubMed
-
- Yatoh S, et al. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56(7):1802–1809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

