Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking
- PMID: 23431141
- PMCID: PMC3593925
- DOI: 10.1073/pnas.1218424110
Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking
Abstract
Parkinson disease and dementia with Lewy bodies are featured with the formation of Lewy bodies composed mostly of α-synuclein (α-Syn) in the brain. Although evidence indicates that the large oligomeric or protofibril forms of α-Syn are neurotoxic agents, the detailed mechanisms of the toxic functions of the oligomers remain unclear. Here, we show that large α-Syn oligomers efficiently inhibit neuronal SNARE-mediated vesicle lipid mixing. Large α-Syn oligomers preferentially bind to the N-terminal domain of a vesicular SNARE protein, synaptobrevin-2, which blocks SNARE-mediated lipid mixing by preventing SNARE complex formation. In sharp contrast, the α-Syn monomer has a negligible effect on lipid mixing even with a 30-fold excess compared with the case of large α-Syn oligomers. Thus, the results suggest that large α-Syn oligomers function as inhibitors of dopamine release, which thus provides a clue, at the molecular level, to their neurotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


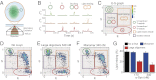
 , emission of donor dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)] excited by donor-excitation laser (green line);
, emission of donor dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)] excited by donor-excitation laser (green line);  , emission of acceptor dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD)] excited by donor-excitation laser, which is FRET signal (orange line); and
, emission of acceptor dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD)] excited by donor-excitation laser, which is FRET signal (orange line); and  , emission of acceptor dye excited by acceptor-excitation laser (red line). Depending on the reaction status, unreacted T and V, docked, and lipid-mixed vesicles have different sets of three fluorescence intensities. (C) Schematic description of the 2D E (FRET efficiency)–S (sorting number) graph. Three fluorescent intensities of a vesicle from the time traces in B are used to calculate E and S (
, emission of acceptor dye excited by acceptor-excitation laser (red line). Depending on the reaction status, unreacted T and V, docked, and lipid-mixed vesicles have different sets of three fluorescence intensities. (C) Schematic description of the 2D E (FRET efficiency)–S (sorting number) graph. Three fluorescent intensities of a vesicle from the time traces in B are used to calculate E and S (

References
-
- Iwai A, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. - PubMed
-
- Clayton DF, George JM. The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21(6):249–254. - PubMed
-
- Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. - PubMed
-
- Irizarry MC, et al. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson’s disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57(4):334–337. - PubMed
-
- Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

