Metabotropic NMDA receptor function is required for β-amyloid-induced synaptic depression
- PMID: 23431156
- PMCID: PMC3593880
- DOI: 10.1073/pnas.1219605110
Metabotropic NMDA receptor function is required for β-amyloid-induced synaptic depression
Abstract
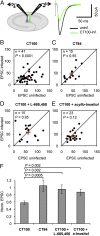
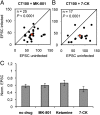
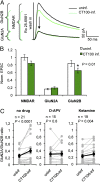
The mechanisms by which β-amyloid (Aβ), a peptide fragment believed to contribute to Alzheimer's disease, leads to synaptic deficits are not known. Here we find that elevated oligomeric Aβ requires ion flux-independent function of NMDA receptors (NMDARs) to produce synaptic depression. Aβ activates this metabotropic NMDAR function on GluN2B-containing NMDARs but not on those containing GluN2A. Furthermore, oligomeric Aβ leads to a selective loss of synaptic GluN2B responses, effecting a switch in subunit composition from GluN2B to GluN2A, a process normally observed during development. Our results suggest that conformational changes of the NMDAR, and not ion flow through its channel, are required for Aβ to produce synaptic depression and a switch in NMDAR composition. This Aβ-induced signaling mediated by alterations in GluN2B conformation may be a target for therapeutic intervention of Alzheimer's disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. - PubMed
-
- Lesné S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. - PubMed
-
- Cheng IH, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282(33):23818–23828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

