Reactive astrocytes secrete lcn2 to promote neuron death
- PMID: 23431168
- PMCID: PMC3593910
- DOI: 10.1073/pnas.1218497110
Reactive astrocytes secrete lcn2 to promote neuron death
Abstract
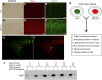
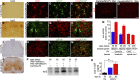
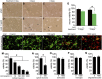
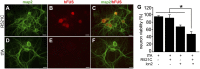
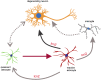
Glial reaction is a common feature of neurodegenerative diseases. Recent studies have suggested that reactive astrocytes gain neurotoxic properties, but exactly how reactive astrocytes contribute to neurotoxicity remains to be determined. Here, we identify lipocalin 2 (lcn2) as an inducible factor that is secreted by reactive astrocytes and that is selectively toxic to neurons. We show that lcn2 is induced in reactive astrocytes in transgenic rats with neuronal expression of mutant human TAR DNA-binding protein 43 (TDP-43) or RNA-binding protein fused in sarcoma (FUS). Therefore, lcn2 is induced in activated astrocytes in response to neurodegeneration, but its induction is independent of TDP-43 or FUS expression in astrocytes. We found that synthetic lcn2 is cytotoxic to primary neurons in a dose-dependent manner, but is innocuous to astrocytes, microglia, and oligodendrocytes. Lcn2 toxicity is increased in neurons that express a disease gene, such as mutant FUS or TDP-43. Conditioned medium from rat brain slice cultures with neuronal expression of mutant TDP-43 contains abundant lcn2 and is toxic to primary neurons as well as neurons in cultured brain slice from WT rats. Partial depletion of lcn2 by immunoprecipitation reduced conditioned medium-mediated neurotoxicity. Our data indicate that reactive astrocytes secrete lcn2, which is a potent neurotoxic mediator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

