Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs
- PMID: 23431169
- PMCID: PMC3607052
- DOI: 10.1073/pnas.1222902110
Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs
Abstract
In the field of regenerative medicine, one of the ultimate goals is to generate functioning organs from pluripotent cells, such as ES cells or induced pluripotent stem cells (PSCs). We have recently generated functional pancreas and kidney from PSCs in pancreatogenesis- or nephrogenesis-disabled mice, providing proof of principle for organogenesis from PSCs in an embryo unable to form a specific organ. Key when applying the principles of in vivo generation to human organs is compensation for an empty developmental niche in large nonrodent mammals. Here, we show that the blastocyst complementation system can be applied in the pig using somatic cell cloning technology. Transgenic approaches permitted generation of porcine somatic cell cloned embryos with an apancreatic phenotype. Complementation of these embryos with allogenic blastomeres then created functioning pancreata in the vacant niches. These results clearly indicate that a missing organ can be generated from exogenous cells when functionally normal pluripotent cells chimerize a cloned dysorganogenetic embryo. The feasibility of blastocyst complementation using cloned porcine embryos allows experimentation toward the in vivo generation of functional organs from xenogenic PSCs in large animals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



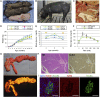
Comment in
-
Literature Watch: implications for transplantation.Am J Transplant. 2013 Jun;13(6):1377. doi: 10.1111/ajt.12322. Am J Transplant. 2013. PMID: 23721549 No abstract available.
References
-
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441(7097):1094–1096. - PubMed
-
- MacLaren RE, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. - PubMed
-
- Kobayashi T, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142(5):787–799. - PubMed
-
- Usui JI, et al. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012;180(6):2417–2426. - PubMed
-
- Sumazaki R, et al. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36(1):83–87. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

