P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia
- PMID: 23431238
- PMCID: PMC3568910
- DOI: 10.1155/2013/271813
P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia
Abstract
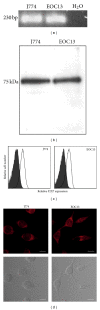
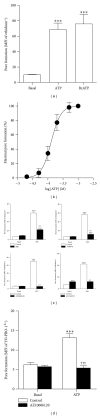
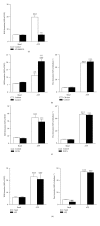
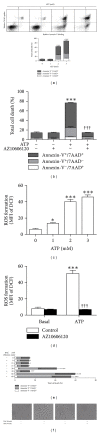
The P2X7 purinergic receptor is a ligand-gated cation channel expressed on leukocytes including microglia. This study aimed to determine if P2X7 activation induces the uptake of organic cations, reactive oxygen species (ROS) formation, and death in the murine microglial EOC13 cell line. Using the murine macrophage J774 cell line as a positive control, RT-PCR, immunoblotting, and immunolabelling established the presence of P2X7 in EOC13 cells. A cytofluorometric assay demonstrated that the P2X7 agonists adenosine-5'-triphosphate (ATP) and 2'(3')-O-(4-benzoylbenzoyl) ATP induced ethidium(+) or YO-PRO-1(2+) uptake into both cell lines. ATP induced ethidium(+) uptake into EOC13 cells in a concentration-dependent manner, with an EC(50) of ~130 μM. The P2X7 antagonists Brilliant Blue G, A438079, AZ10606120, and AZ11645373 inhibited ATP-induced cation uptake into EOC13 cells by 75-100%. A cytofluorometric assay demonstrated that P2X7 activation induced ROS formation in EOC13 cells, via a mechanism independent of Ca(2+) influx and K(+) efflux. Cytofluorometric measurements of Annexin-V binding and 7AAD uptake demonstrated that P2X7 activation induced EOC13 cell death. The ROS scavenger N-acetyl-L-cysteine impaired both P2X7-induced EOC13 ROS formation and cell death, suggesting that ROS mediate P2X7-induced EOC13 death. In conclusion, P2X7 activation induces the uptake of organic cations, ROS formation, and death in EOC13 microglia.
Figures



References
-
- Nimmerjahn A, Kirchhoff F, Helmchen F. Neuroscience: resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo . Science. 2005;308(5726):1314–1318. - PubMed
-
- Helmut K, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological Reviews. 2011;91(2):461–553. - PubMed
-
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience. 2007;10(11):1387–1394. - PubMed
-
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annual Review of Immunology. 2009;27:119–145. - PubMed
-
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Progress in Neurobiology. 2005;76(2):77–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

