Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis
- PMID: 23431276
- PMCID: PMC3561183
- DOI: 10.1371/journal.ppat.1003120
Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis
Abstract
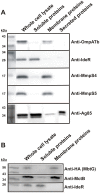
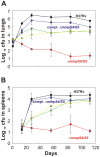
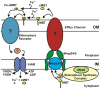
Iron is an essential nutrient for most bacterial pathogens, but is restricted by the host immune system. Mycobacterium tuberculosis (Mtb) utilizes two classes of small molecules, mycobactins and carboxymycobactins, to capture iron from the human host. Here, we show that an Mtb mutant lacking the mmpS4 and mmpS5 genes did not grow under low iron conditions. A cytoplasmic iron reporter indicated that the double mutant experienced iron starvation even under high-iron conditions. Loss of mmpS4 and mmpS5 did not change uptake of carboxymycobactin by Mtb. Thin layer chromatography showed that the ΔmmpS4/S5 mutant was strongly impaired in biosynthesis and secretion of siderophores. Pull-down experiments with purified proteins demonstrated that MmpS4 binds to a periplasmic loop of the associated transporter protein MmpL4. This interaction was corroborated by genetic experiments. While MmpS5 interacted only with MmpL5, MmpS4 interacted with both MmpL4 and MmpL5. These results identified MmpS4/MmpL4 and MmpS5/MmpL5 as siderophore export systems in Mtb and revealed that the MmpL proteins transport small molecules other than lipids. MmpS4 and MmpS5 resemble periplasmic adapter proteins of tripartite efflux pumps of Gram-negative bacteria, however, they are not only required for export but also for efficient siderophore synthesis. Membrane association of MbtG suggests a link between siderophore synthesis and transport. The structure of the soluble domain of MmpS4 (residues 52-140) was solved by NMR and indicates that mycobacterial MmpS proteins constitute a novel class of transport accessory proteins. The bacterial burden of the mmpS4/S5 deletion mutant in mouse lungs was lower by 10,000-fold and none of the infected mice died within 180 days compared to wild-type Mtb. This is the strongest attenuation observed so far for Mtb mutants lacking genes involved in iron utilization. In conclusion, this study identified the first components of novel siderophore export systems which are essential for virulence of Mtb.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Searle S, Bright NA, Roach TI, Atkinson PG, Barton CH, et al. (1998) Localisation of Nramp1 in macrophages: modulation with activation and infection. J Cell Sci 111: 2855–2866. - PubMed
-
- Cellier MF, Courville P, Campion C (2007) Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect 9: 1662–1670. - PubMed
-
- Wagner D, Maser J, Lai B, Cai Z, Barry CE 3rd, et al. (2005) Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J Immunol 174: 1491–1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

