Morinda citrifolia (Noni) as an Anti-Inflammatory Treatment in Women with Primary Dysmenorrhoea: A Randomised Double-Blind Placebo-Controlled Trial
- PMID: 23431314
- PMCID: PMC3569913
- DOI: 10.1155/2013/195454
Morinda citrifolia (Noni) as an Anti-Inflammatory Treatment in Women with Primary Dysmenorrhoea: A Randomised Double-Blind Placebo-Controlled Trial
Abstract
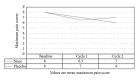
Introduction. Noni (Morinda citrifolia) has been used for many years as an anti-inflammatory agent. We tested the efficacy of Noni in women with dysmenorrhea. Method. We did a prospective randomized double-blind placebo-controlled trial in 100 university students of 18 years and older over three menstrual cycles. Patients were invited to participate and randomly assigned to receive 400 mg Noni capsules or placebo. They were assessed for baseline demographic variables such as age, parity, and BMI. They were also assessed before and after treatment, for pain, menstrual blood loss, and laboratory variables: ESR, hemoglobin, and packed cell volume. Results. Of the 1027 women screened, 100 eligible women were randomized. Of the women completing the study, 42 women were randomized to Noni and 38 to placebo. There were no significant differences in any of the variables at randomization. There were also no significant differences in mean bleeding score or pain score at randomization. Both bleeding and pain scores gradually improved in both groups as the women were observed over three menstrual cycles; however, the improvement was not significantly different in the Noni group when compared to the controls. Conclusion. Noni did not show a reduction in menstrual pain or bleeding when compared to placebo.
Figures
References
-
- French L. Dysmenorrhea. American Family Physician. 2005;71(2):285–291. - PubMed
-
- French L. Dysmenorrhea in adolescents: diagnosis and treatment. Pediatric Drugs. 2008;10(1):1–7. - PubMed
-
- Ylikorkala O, Dawood MY. New concepts in dysmenorrhea. American Journal of Obstetrics and Gynecology. 1978;130(7):833–847. - PubMed
-
- Powell AM, Chan WY, Alvin P, Litt IF. Menstrual-PGF2α, PGE2 and TXA2 in normal and dysmenorrheic women and their temporal relationship to dysmenorrhea. Prostaglandins. 1985;29(2):273–290. - PubMed
-
- Hayes EC, Rock JA. COX-2 inhibitors and their role in gynecology. Obstetrical and Gynecological Survey. 2002;57(11):768–780. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous