Morphological and metabolic changes in the nigro-striatal pathway of synthetic proteasome inhibitor (PSI)-treated rats: a MRI and MRS study
- PMID: 23431380
- PMCID: PMC3576393
- DOI: 10.1371/journal.pone.0056501
Morphological and metabolic changes in the nigro-striatal pathway of synthetic proteasome inhibitor (PSI)-treated rats: a MRI and MRS study
Abstract
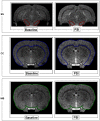
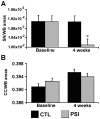
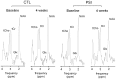
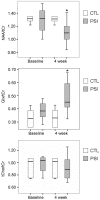
Systemic administration of a Synthetic Proteasome Inhibitor (PSI) in rats has been described as able to provide a model of Parkinson's disease (PD), characterized by behavioral and biochemical modifications, including loss of dopaminergic neurons in the substantia nigra (SN), as assessed by post-mortem studies. With the present study we aimed to assess in-vivo by Magnetic Resonance (MR) possible morphological and metabolic changes in the nigro-striatal pathway of PSI-treated rats. 10 animals were subcutaneously injected with PSI 6.0 mg/kg dissolved in DMSO 100%. Injections were made thrice weekly over the course of two weeks. 5 more animals injected with DMSO 100% with the same protocol served as controls. The animals underwent MR sessions before and at four weeks after the end of treatment with either PSI or vehicle. MR Imaging was performed to measure SN volume and Proton MR Spectroscopy ((1)H-MRS) was performed to measure metabolites changes at the striatum. Animals were also assessed for motor function at baseline and at 4 and 6 weeks after treatment. Dopamine and dopamine metabolite levels were measured in the striata at 6 weeks after treatment. PSI-treated animals showed volumetric reduction of the SN (p<0.02) at 4 weeks after treatment as compared to baseline. Immunofluorescence analysis confirmed MRI changes in SN showing a reduction of tyrosine hydroxylase expression as compared to neuron-specific enolase expression. A reduction of N-acetyl-aspartate/total creatine ratio (p = 0.05) and an increase of glutamate-glutamine-γ amminobutirrate/total creatine were found at spectroscopy (p = 0.03). At 6 weeks after treatment, PSI-treated rats also showed motor dysfunction compared to baseline (p = 0.02), accompanied by dopamine level reduction in the striatum (p = 0.02). Treatment with PSI produced morphological and metabolic modifications of the nigro-striatal pathway, accompanied by motor dysfunction. MR demonstrated to be a powerful mean to assess in-vivo the nigro-striatal pathway morphology and metabolism in the PSI-based PD animal model.
Conflict of interest statement
Figures



References
-
- Dauer W (2003) Przedborski (2003) Parkinson’s disease: mechanisms and models. Neuron 39: 889–909. - PubMed
-
- McNaught KS, Perl DP, Brownell AL, Olanow CW (2004) Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol 56: 149–162. - PubMed
-
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH (2009) Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis 35: 385–398. - PubMed
-
- Schapira AH, Cleeter MW, Muddle JR, Workman JM, Cooper JM, et al. (2006) Proteasomal inhibition causes loss of nigral tyrosine hydroxylase neurons. Ann Neurol 60: 253–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

