Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement
- PMID: 23432593
- PMCID: PMC3755729
- DOI: 10.1111/add.12127
Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement
Abstract
Aims: In HIV-infected individuals, heavy drinking compromises survival. In HIV primary care, the efficacy of brief motivational interviewing (MI) to reduce drinking is unknown, alcohol-dependent patients may need greater intervention and resources are limited. Using interactive voice response (IVR) technology, HealthCall was designed to enhance MI via daily patient self-monitoring calls to an automated telephone system with personalized feedback. We tested the efficacy of MI-only and MI+HealthCall for drinking reduction among HIV primary care patients.
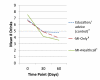
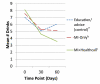
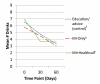
Design: Parallel random assignment to control (n = 88), MI-only (n = 82) or MI+HealthCall (n = 88). Counselors provided advice/education (control) or MI (MI-only or MI+HealthCall) at baseline. At 30 and 60 days (end-of-treatment), counselors briefly discussed drinking with patients, using HealthCall graphs with MI+HealthCall patients.
Setting: Large urban HIV primary care clinic.
Participants: Patients consuming ≥4 drinks at least once in prior 30 days.
Measurements: Using time-line follow-back, primary outcome was number of drinks per drinking day, last 30 days.
Findings: End-of-treatment number of drinks per drinking day (NumDD) means were 4.75, 3.94 and 3.58 in control, MI-only and MI+HealthCall, respectively (overall model χ(2) , d.f. = 9.11,2, P = 0.01). For contrasts of NumDD, P = 0.01 for MI+HealthCall versus control; P = 0.07 for MI-only versus control; and P = 0.24 for MI+HealthCall versus MI-only. Secondary analysis indicated no intervention effects on NumDD among non-alcohol-dependent patients. However, for contrasts of NumDD among alcohol-dependent patients, P < 0.01 for MI+HealthCall versus control; P = 0.09 for MI-only versus control; and P = 0.03 for MI+HealthCall versus MI-only. By 12-month follow-up, although NumDD remained lower among alcohol-dependent patients in MI+HealthCall than others, effects were no longer significant.
Conclusions: For alcohol-dependent HIV patients, enhancing MI with HealthCall may offer additional benefit, without extensive additional staff involvement.
Trial registration: ClinicalTrials.gov NCT00371969.
© 2013 The Authors, Addiction © 2013 Society for the Study of Addiction.
Figures



References
-
- Rosenthal E, Salmon-Ceron D, Lewden C, Bouteloup V, Pialoux G, Bonnet F, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalite 2005 survey, ANRS EN19) HIV Med. 2009;10:282–9. - PubMed
-
- Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198–209. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

