Optimal specimen collection and transport methods for the detection of avian influenza virus and Newcastle disease virus
- PMID: 23432911
- PMCID: PMC3599916
- DOI: 10.1186/1746-6148-9-35
Optimal specimen collection and transport methods for the detection of avian influenza virus and Newcastle disease virus
Abstract
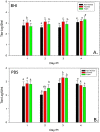
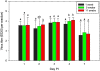
Background: Active and passive surveillance for avian influenza virus (AIV) and Newcastle disease virus (NDV) is widespread in commercial poultry worldwide, therefore optimization of sample collection and transport would be valuable to achieve the best sensitivity and specificity possible, and to develop the most accurate and efficient testing programs. A H7N2 low pathogenicity (LP) AIV strain was selected and used as an indicator virus because it is present in lower concentrations in swabbings and thus requires greater sensitivity for detection compared to highly pathogenic (HP) AIV. For similar reasons a mesogenic strain of NDV was selected. Using oro-pharyngeal and cloacal swabs collected from chickens experimentally exposed to the viruses we evaluated the effects of numerous aspects of sample collection and transport: 1) swab construction material (flocked nylon, non-flocked Dacron, or urethane foam), 2) transport media (brain heart infusion broth [BHI] or phosphate buffered saline [PBS]), 3) media volume (2 ml or 3.5 ml), 4) transporting the swab wet in the vial or removing the swab prior to transport, or transporting the swab dry with no media, and 5) single swabs versus pooling 5 or 11 swabs per vial.
Results: Using real-time RT-PCR (rRT-PCR), virus isolation (VI) and commercial antigen detection immunoassays for AIV we observed statistically significant differences and consistent trends with some elements of sample collection and transport; media, dry transport and swab construction. Conversely, the number of swabs pooled (1, 5 or 11) and whether the swab was removed prior to transport did not impact virus detection. Similarly, with NDV detection by both VI and rRT-PCR was not affected by the numbers of swabs collected in a single vial (1, 5 or 11).
Conclusions: We observed that flocked and foam swabs were superior to non-flocked swabs, BHI media was better than PBS, and transporting swabs wet was better for virus recovery and detection than transporting them dry. There was no observable difference in detection whether the swab was removed prior to transport or left in the vial. Also, with both AIV and NDV, there was no observed difference in virus detection between pools of 1, 5 or 11 swabs.
Figures





References
-
- Roelandt S, Outtrim L, Browning C, Alexander DJ, Brown IH, Irvine RM. Evaluation of two different swab transport systems in the detection of avian influenza virus excretion from infected Pekin ducks (Anas platyrhynchos) J Virol Methods. 2012;184(1–2):8–14. - PubMed
-
- Scansen KA, Bonsu BK, Stoner E, Mack K, Salamon D, Leber A, Marcon MJ. Comparison of polyurethane foam to nylon flocked swabs for collection of secretions from the anterior nares in performance of a rapid influenza virus antigen test in a pediatric emergency department. J Clin Microbiol. 2010;48(3):852–856. doi: 10.1128/JCM.01897-09. - DOI - PMC - PubMed
-
- Esposito S, Molteni CG, Daleno C, Valzano A, Cesati L, Gualtieri L, Tagliabue C, Bosis S, Principi N. Comparison of nasopharyngeal nylon flocked swabs with universal transport medium and rayon-bud swabs with a sponge reservoir of viral transport medium in the diagnosis of paediatric influenza. J Med Microbiol. 2010;59(Pt 1):96–99. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

