The 'ubiquitous' reality of vector immunology
- PMID: 23433059
- PMCID: PMC3898176
- DOI: 10.1111/cmi.12128
The 'ubiquitous' reality of vector immunology
Abstract
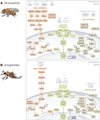
Ubiquitination (ubiquitylation) is a common protein modification that regulates a multitude of processes within the cell. This modification is typically accomplished through the covalent binding of ubiquitin to a lysine residue onto a target protein and is catalysed by the presence of three enzymes: an activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin-protein ligase (E3). In recent years, ubiquitination has risen as a major signalling regulator of immunity and microbial pathogenesis in the mammalian system. Still, little is known about how ubiquitin relates specifically to vector immunology. Here, we provide a brief overview of ubiquitin biochemistry and describe how ubiquitination regulates immune responses in arthropods of medical relevance. We also discuss scientific gaps in the literature and suggest that, similar to mammals, ubiquitin is a major regulator of immunity in medically important arthropods.
© 2013 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. - PubMed
-
- Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42:779–789. - PubMed
-
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. - PubMed
-
- Arjona A, Ledizet M, Anthony K, Bonafe N, Modis Y, Town T, Fikrig E. West Nile virus envelope protein inhibits dsRNA-induced innate immune responses. J Immunol. 2007;179:8403–8409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

